Research
The Bennett lab studies broad aspects of fungal biology, including genetic and epigenetic mechanisms of phenotypic variation in Candida species and their impact on both commensalism and pathogenesis.
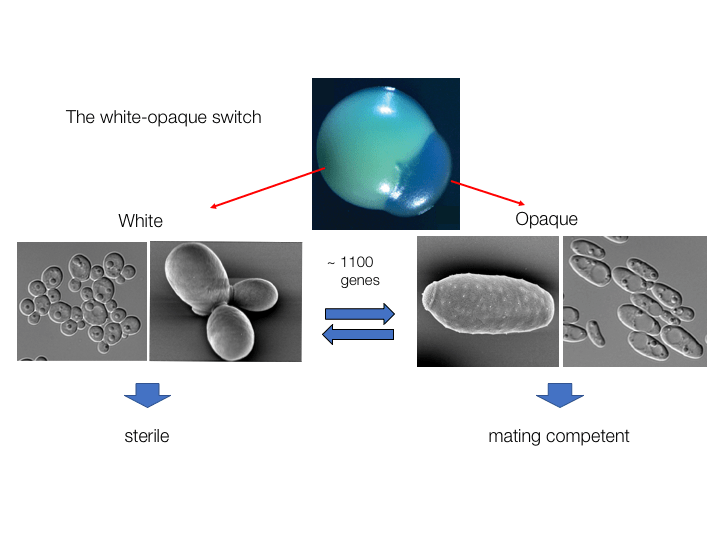
Epigenetic Switching
Candida species can undergo “phenotypic switching” in which cells exhibit heritable and reversible transitions between different cell states. This is best exemplified by the white-opaque switch in C. albicans which involves rewiring of transcriptional networks in combination with changes in chromatin structure. Analysis of the switch can provide fundamental insights into how eukaryotic cells undergo heritable changes in cell state.
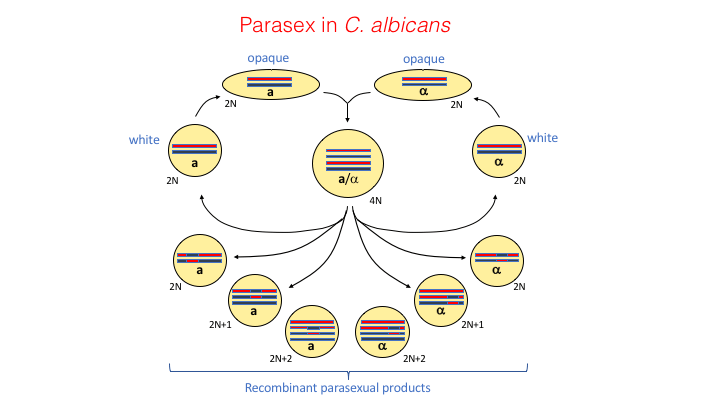
Parasexual Reproduction
The lab has a long-standing interest in the sexual cycles of Candida species. This has led to the identification of specialized mating cycles including the identification of same-sex mating in Candida albicans. This species lacks a conventional meiosis and instead undergoes a program of concerted chromosome loss (CCL) to complete a ‘parasexual’ cycle
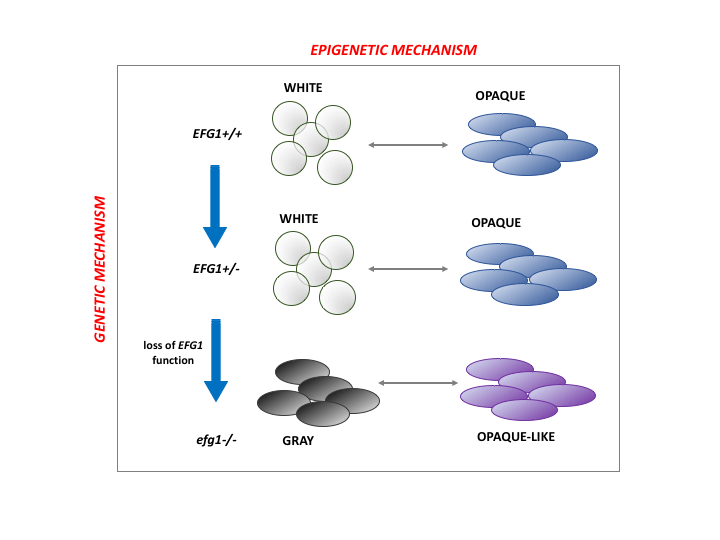
phenotypic variation
High-frequency genetic events can increase phenotypic variation in C. albicans. The “white-to-gray” switch occurs in diploid strains that are naturally heterozygous for a key transcription factor. Loss of heterozygosity events produce a null mutant – the gray state – that exhibits increased fitness in certain gastrointestinal models of commensal infection.
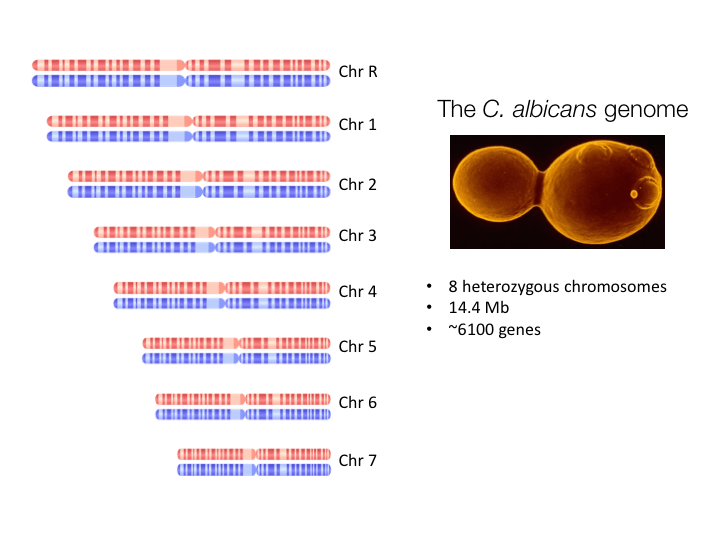
GENOMe ANALYSIS
Many pathogenic Candida species show extensive plasticity due to a variety of mutational and recombinational processes. We study C. albicans genomes from clinical isolates to identify genetic loci responsible for differences in clinically-relevant traits. We also perform experimental evolution experiments in which C. albicans strains are passaged and re-sequenced to determine how the genome changes over short time scales and how this influences interactions with the host.