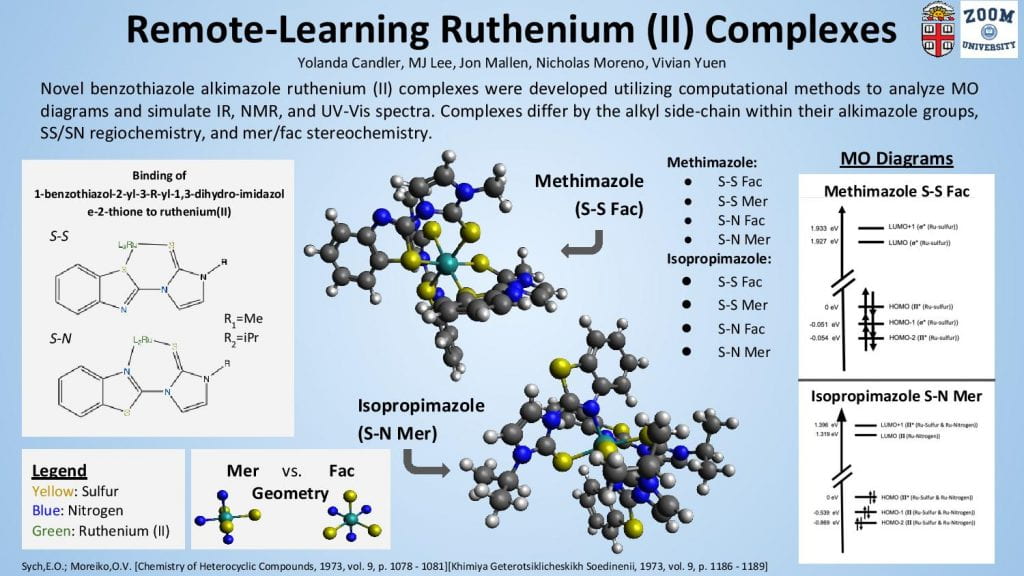
Class: CHEM 0500 – Inorganic Chemistry
Instructor(s): Dr. Eric Victor
Student(s): Keerthi Sreenivasan (Chemistry ’21), Sophia Zheng, Laura Perlmutter, Allison Lin, Veronica Gordon
“This poster includes generated data calculations and analysis of the notable properties of the ligands as they change after bonding to the Ru metal center.”
–Keerthi Sreenivasan
Description:
Based on an existing protocol for synthesizing 2,6-bis[(S)-isopropyl-4,5-dihydro-oxazol-2-yl]- thiobenzene by Peer et. al, we tested a novel microwave synthesis protocol aimed at shortening the 3-5 day synthesis time. After two rounds of testing microwave synthesis, characterization of the product using GC-MS was inconclusive, and future refinement of the microwave synthesis protocol is necessary. Due to the transition to remote work, we then moved to computational work on the ligand and two modified versions. Calculations were performed to determine structural parameters of the ligands, simulations of spectroscopy and orbital images. A second round of calculations was done while binding the ligands in a tridentate fashion to a ruthenium metal complex. Reactive nitrogen oxide species have been shown to play a significant role in biological processes, and various metal complexes show reactivity with SNO and SSNO anions. In this CURE project, we were able to learn how to perform novel synthetic methods for a ruthenium complex using published ligands and characterize them using computational software.