Brown Multimodal Imaging of Cognition, Aging, and Alzheimer’s Disease (MICAAD) Study

An accumulation of beta-amyloid (Aβ) peptides in the brain is one of the pathological hallmarks of Alzheimer’s disease and presents a high risk of developing the disease. Recent advances in in vivo positron emission tomography (PET) have significantly increased our awareness of the presence of Aβ plaques in approximately 20-50% of asymptomatic older adults who are now considered as preclinical Alzheimer’s disease. Differentiating healthy older adults from those who are in the preclinical stage of Alzheimer’s disease remains challenging, in part because we lack sensitive and specific behavioral and neural measures that detect the presence of Aβ plaques in the preclinical Alzheimer’s disease stage. Furthermore, the co-presence of tau-protein neurofibrillary tangles (NFT), another key pathological marker of Alzheimer’s disease, makes it difficult to link Alzheimer’s pathologies to specific cognitive and brain network dysfunction.
The Brown MICAAD Study aims to examine how normal brain aging and Alzheimer’s disease pathology affect cognition, with emphasis on visual memory, and the underlying neural systems in clinically intact older adults with and without Alzheimer’s pathologies. Under the support of an award from the National Institutes of Health (NIH R01 AG068990), the study enrolls 50 healthy young adults and 125 clinically intact older adults using multimodal neuroimaging approaches that include the-state-of-the-field PET scans that image brain amyloid and tau pathologies and structural and functional MRI. In combination with novel cognitive assessments and neural network analyses of the neuroimaging measurements, the study aims to identify more sophisticated behavioral phenotypes and neural markers at the early stage of Alzheimer’s disease pathologies. These findings will reveal mechanistic explanations of the functional networks underlying visual memory processes and the impact of normal aging and Alzheimer’s disease pathologies on these networks. Furthermore, the study outcomes will enable a non-invasive and more affordable and accessible screening test of the disease with clinical utility in aiding early diagnosis and treatment monitoring.
Longitudinal Multicenter Head-to-Head Harmonization of tau PET tracers (HEAD Study)
Tau neurofibrillary tangle accumulation is another key neuropathological feature of Alzheimer’s disease, besides brain beta-amyloid plaques. Tau pathology accumulates in parts of the brain different from those where amyloid plaques form in the early stage of Alzheimer’s disease and has been linked closer to cognitive changes. Tracking longitudinal tau accumulation in living people, thus, is crucial for a better understanding of the natural history of tau pathology, monitoring cognitive changes and disease progression, and testing drug effects on tau accumulation in clinical trials. Several tau PET tracers have been developed in recent years, but these tau PET tracers have distinct affinity to tau tangles and present different characteristic signals.
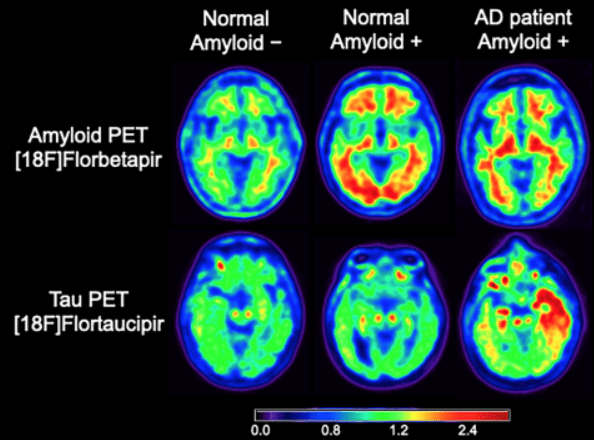
The HEAD Study, a multi-center longitudinal project funded by the National Institutes of Health (NIH R01 AG073267), aims to examine different characteristics associated with tau PET tracers and improve generalization of findings obtained in studies and clinical practice using distinct tau tracers. The study will recruit 620 healthy young, cognitively normal older adults and patients with Alzheimer’s disease who will undergo amyloid and tau PET scans, along with MRI scans, at two time points. These imaging markers will be related to a set of cognitive measurements. The study findings will shed light on the aforementioned issues, evaluating similarities and differences of the tau imaging agents towards the harmonization of cross-sectional and longitudinal tau PET results and their impact on cognition along the spectrum of Alzheimer’s disease.
Top-down and Bottom-up Pathways of Visual Scene Memory across the Lifespan
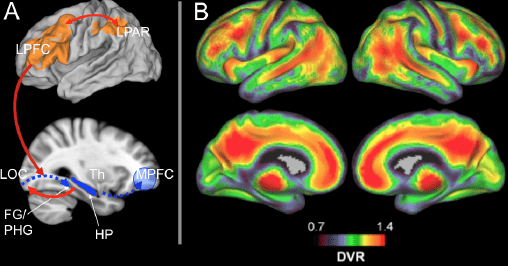
The goal of the study is to elucidate the neural pathways and cognitive mechanisms of human visual memory and the source of changes in visual memory during normal and pathological brain aging processes. Emerging evidence suggests that a dynamic interaction between attention, perception, and memory occurs to give rise to visual scene representations in memory. As such, visual scene memory relies on multiple brain structures involved in perceptual bottom-up (“feedforward”) to higher-level top-down (“feedback”) processing of visual scenes in the memory process. While activation patterns for visual scene images within cortical/subcortical networks have been studied extensively, it remains largely unknown how these brain regions interact and what specific role they play in the service of visual scene memory. Moreover, the cognitive aging literature indicates selective vulnerability of visual scene memory to age and beta-amyloid deposition, a pathological hallmark of Alzheimer’s disease (AD), among cognitively normal older adults, representing a stage of disease now referred to as “preclinical AD”. However, little research has explored how the interaction across these brain regions as the source of feedforward and feedback connections changes in brain aging and Alzheimer’s disease pathology. Using novel behavioral paradigms, multimodal in-vivo neuroimaging, and advanced multivariate analyses, the study tests the hypothesized dynamics of this neural network underlying visual scene memory, and then will determine how brain aging and Alzheimer’s disease pathology influence this system and visual scene memory performance. (Funding source: NIGMS/Brown Carney Institute P20 GM103645)
Aging, Beta-Amyloid, and Memory Networks
Although cognitively normal, brain aging in older adults occurs in a highly heterogeneous manner: it undergoes a wide range of abnormal neural changes that are not clinically evident. One of these abnormal neural changes is an accumulation of beta-amyloid (Aβ), a pathological hallmark of Alzheimers disease (AD). Brain systems supporting memory were shown to be particularly vulnerable to the effect of Aβ deposition. Using amyloid PET, glucose metabolism PET, and functional magnetic resonance imaging (fMRI), we demonstrated that differential neural plasticity occurs in response to aging and Aβ deposition in cognitively normal elderly in both regional activity and functional synchrony across the frontoparietal control regions and medial temporal lobes (right parahippocampal gyrus [rPHG]) implicated in episodic memory.
Hwamee Oh & William J. Jagust (2013). Frontotemporal network connectivity during memory encoding is increased with aging and disrupted by beta-amyloid. The Journal of Neuroscience, 33(47), 18425-37.
Hwamee Oh, Christian Habeck, Cindee Madison, William J. Jagust (2014). Covarying patterns of Abeta deposition, glucose metabolism, and gray matter volume in cognitively normal elderly. Human Brain Mapping, 35(1): 297-308.
*Jeremy Elman, *Hwamee Oh, Cindee M. Madison, Suzanne L. Baker, Jacob W. Vogel, Shawn M. Marks, Sam Crowley, James P. ONeil, William J. Jagust (2014). Neural compensation in older people with brain Abeta deposition. Nature Neuroscience, 2014; 17(10): 1316-8.*Co-First Authors.
Hwamee Oh, Jason Steffener, Ray Razlighi, Christian Habeck, Dan Liu, Yunglin Gazes, Sarah Janicki, Yaakov Stern (2015). Abeta-related hyperactivation in fronto-parietal control regions in cognitively normal elderly. Neurobiology of Aging 36(12), 3247-54.
Hwamee Oh, Jason Steffener, Ray Razlighi, Christian Habeck, Yaakov Stern. (2016). Beta-amyloid deposition is associated with decreased right prefrontal activation during task switching among cognitively normal elderly. The Journal of Neuroscience, 36(6), 1962-70.
Hwamee Oh, Cindee M. Madison, Suzanne L. Baker, Gil Rabinovici, William J. Jagust (2016). Dynamic relationships between age, beta-amyloid deposition, and glucose metabolism link to the regional vulnerability to Alzheimers disease. Brain, 139 (8): 2275-2289.
Neuromorphological Changes with Aging and Beta-Amyloid Deposition
Not only functional alterations described above but also neuromorphological changes occur with aging and Aβ deposition. In a set of studies, we examined changes in brain structure using gray matter volume and cortical thickness. Independent of Aβ deposition, a substantial aging-related reduction in gray matter volume and cortical thickness was found in most of brain regions, while Aβ-related gray matter atrophy was additionally found in frontal and parietal cortices and hippocampus [Figure]. Among these regions undergoing gray matter atrophy, contributions of gray matter volume to cognition in older adults were more regionally specific, but the structure-cognition relationship was different dependent upon the level of Aβ deposition: A fronto-striatal network was related to cognition in older adults without Aβ deposition, while a hippocampal network was related to cognition in older adults with Aβ deposition. These findings contribute to a growing movement within cognitive aging research to better understand aging-related neural constraints that are driven by more heterogeneous etiologies than previously thought and their differential impact on cognition.
Hwamee Oh, Elizabeth C. Mormino, Cindee Madison, Amynta Hayenga, Andre Smiljic, William J. Jagust (2011). Beta-amyloid affects frontal and posterior brain networks in normal aging. NeuroImage, 54(3), 1887-1895.
Miranka Wirth, Sylvia Villeneuve, Claudia M. Haase, Cindee Madison,Hwamee Oh, Susan Landau, Gil Rabinovici, William J. Jagust (2013). Associations between Alzheimer’s disease biomarkers, neurodegeneration, and cognition in normal older people. JAMA Neurology, 70(12):1512-9.
Hwamee Oh, Cindee Madison, Sylvia Villeneuve, Candace Markley, William J. Jagust (2014). Association of gray matter atrophy with age, beta-amyloid, and cognition in aging. Cerebral Cortex, 24(6): 1609-18.
Qolamreza Razlighi, Hwamee Oh, David Parker, Christian Habeck, Yaakov Stern. (2017). Dynamic patterns of brain structure-behavior correlation across the lifespan. Cerebral Cortex, 27(7), 3586-99.
Cognitive Changes with Aging and Beta-Amyloid Deposition
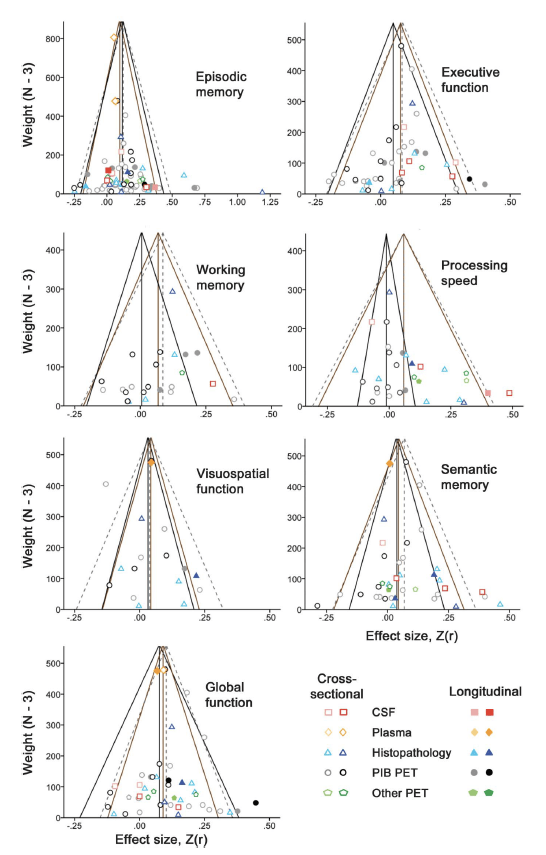
Considering that a relatively large proportion of cognitively normal elderly presents with Aβ deposition, it is not clear to what extent age and Aβ deposition contribute to cognitive changes in normal older adults. In a series of studies, we found that regardless of Aβ deposition, older adults, compared to young controls, show poorer performance in visual episodic memory and executive functions while performance in verbal episodic memory and semantic memory is preserved. Aβ-positive older adults, however, showed greater cognitive decline compared to Aβ-negative older adults, suggesting that even within a normal range of cognition, Aβ deposition is detrimentally associated with cognitive functions in normal older adults. In collaboration with researchers at Harvard/MGH, we further showed a significantly negative relationship between amyloid and cognition in a meta-analysis conducted on a large sample (7140 subjects) collapsing across 64 published studies.
Hwamee Oh, Cindee Madison, Thaddeus J. Haight, Candace Markley, William J. Jagust (2012). Effects of age and beta-amyloid on cognitive changes in normal elderly people. Neurobiology of Aging, 33(12), 2746-55.
Trey Hedden, Hwamee Oh, Alayna P. Younger, and Tanu Patel (2013). Meta-Analysis of Amyloid-Cognition Relations in Cognitively Normal Older Adults. Neurology, 80(14), 1341-8.
Individual Difference and Lifestyle Factors Moderating Brain Aging and Beta-Amyloid Deposition
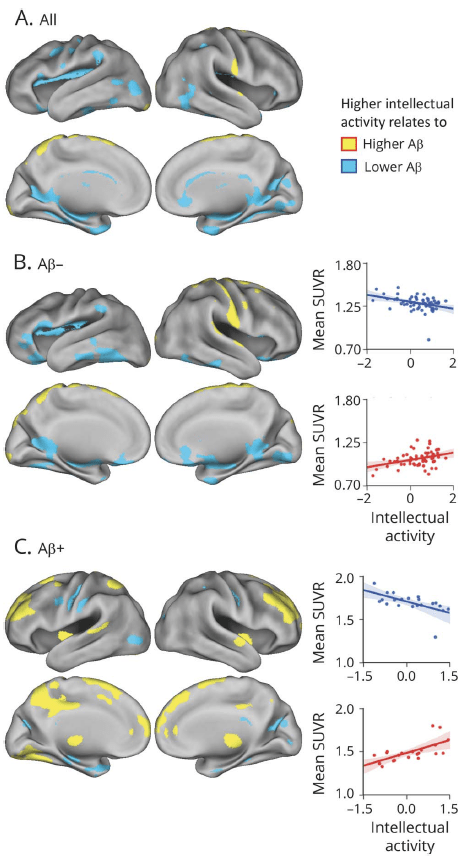
A degree of the relationship between brain aging and cognition, however, seems to vary across individuals. In a set of studies, we showed that the observed relationship between Aβ deposition and cognition was moderated by a level of neural integrity such as metabolic rates and that cognitively stimulating activities across the lifetime affect the degree of AD pathology during aging.
Hwamee Oh, Qolamreza R. Razlighi, Yaakov Stern (2018). Multiple pathways of reserve simultaneously present in cognitively normal older adults. Neurology, 90(3): 197-205.
Miranka Wirth, Hwamee Oh, Beth Mormino, Candace Markley, Susan Landau, William J. Jagust (2013). The effect of beta-amyloid on cognitive decline is modulated by neural integrity in cognitively normal elderly. Alzheimers & Dementia, 9(6): 687-698.
Rik Ossenkoppele, Cindee Madison, Hwamee Oh, Miranka Wirth, Bart N.M. van Berckel, William J. Jagust (2014). Is Verbal Episodic Memory in Elderly with Amyloid Deposits Preserved Through Altered Neuronal Function? Cerebral Cortex, 24(8): 2210-8.
Susan M. Landau, Shawn M. Marks, Elizabeth C. Mormino, Gil D. Rabinovici, Hwamee Oh, Robert S. Wilson, William J. Jagust (2012). Association of lifetime cognitive engagement and low beta-amyloid deposition. JAMA Neurology, 69(5), 623-29.
Copyright Notice: The documents distributed here have been provided as a means to ensure timely dissemination of scholarly and technical work on a noncommercial basis. Copyright and all rights therein are maintained by the authors or by other copyright holders, notwithstanding that they have offered their works here electronically.