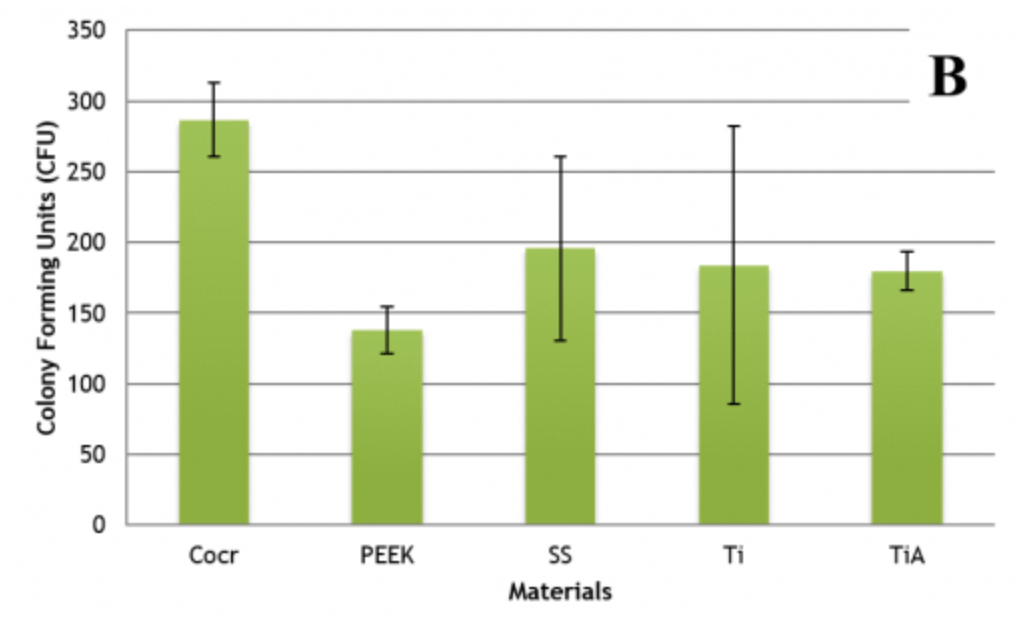
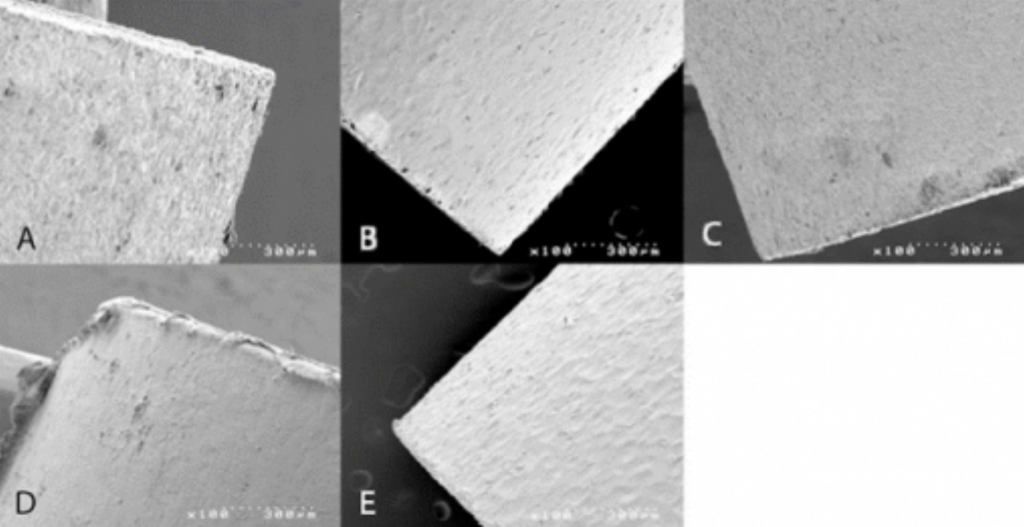
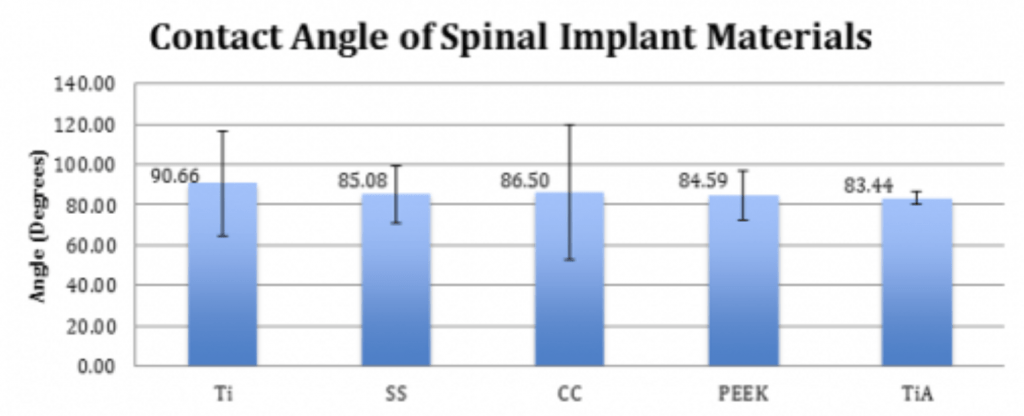
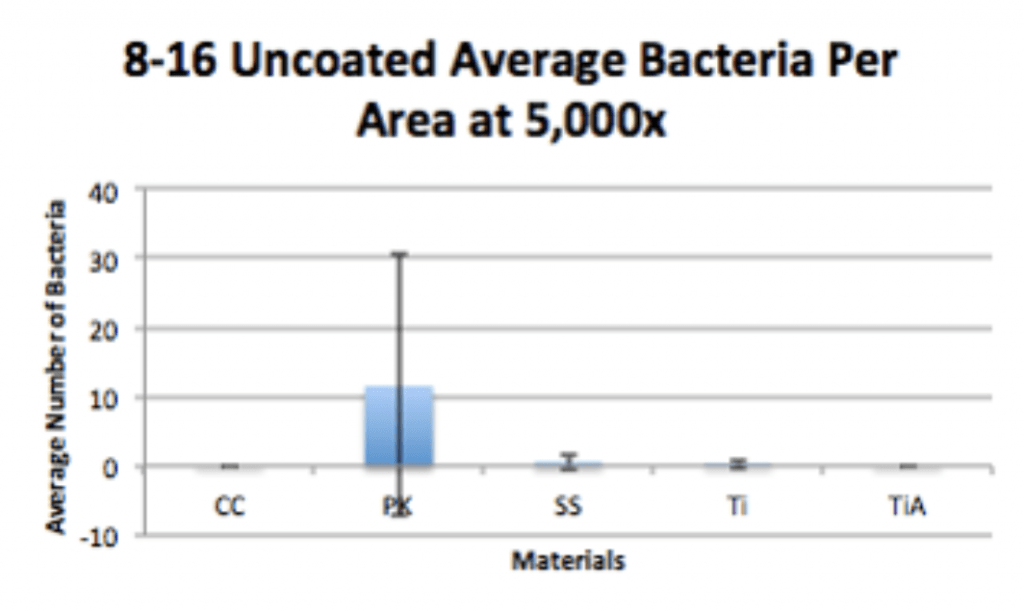
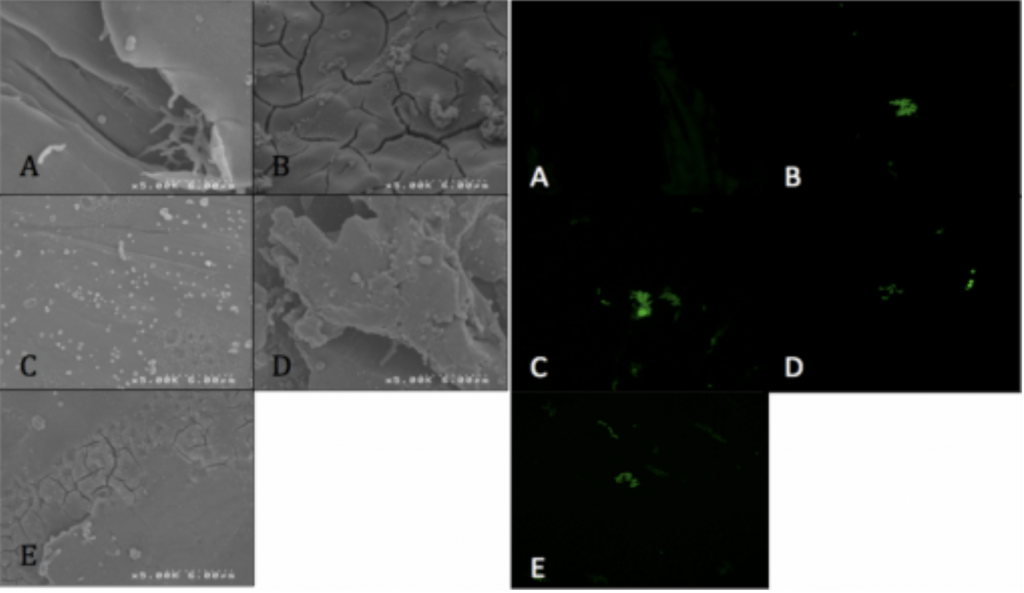
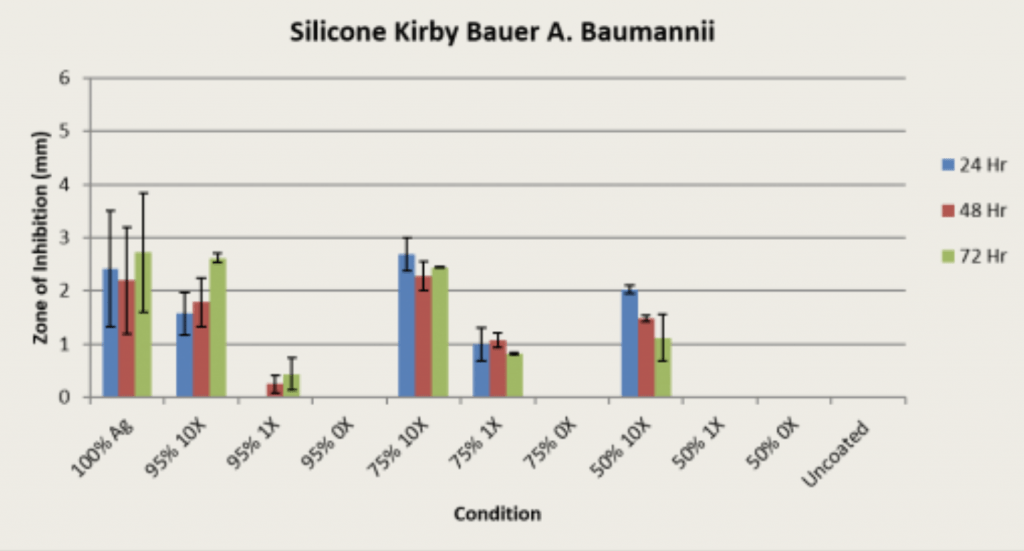
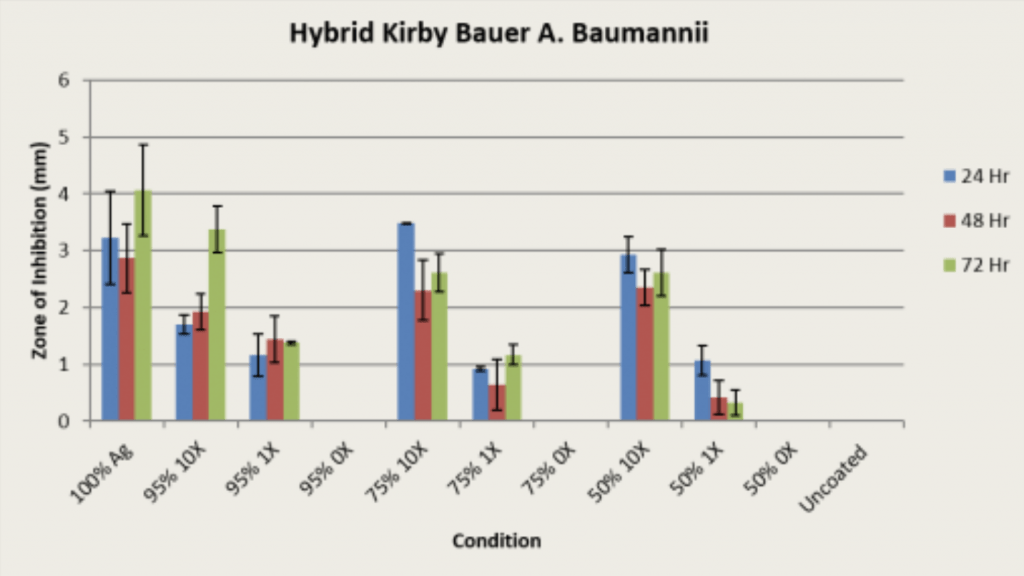
Methodology
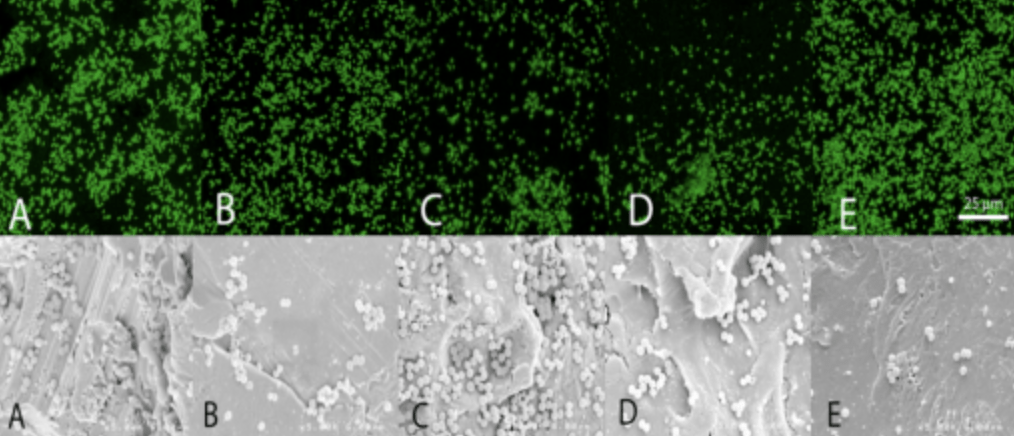
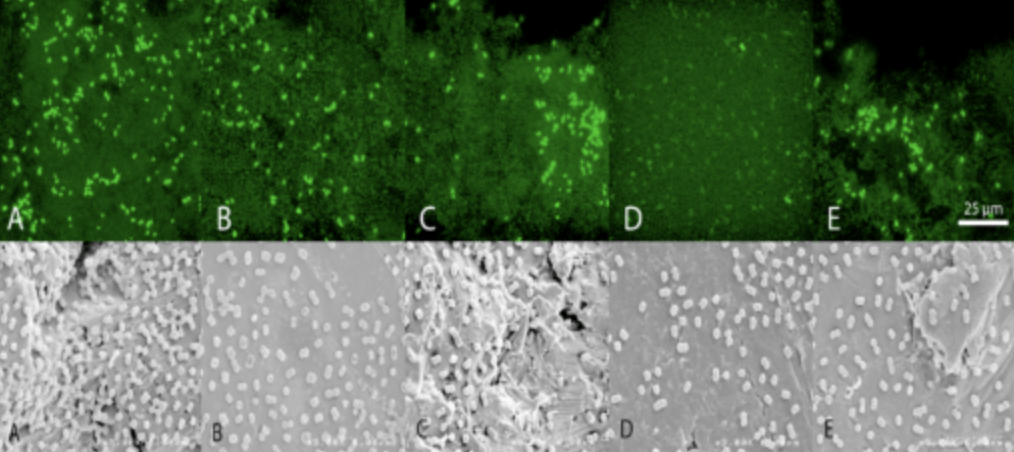
- Confocal Laser-Scanning microscopy and conjugated fluorescent antibodies.
- Visualize and quantify microbial adherence directly on orthopaedic explants.
- Optical Sectioning of Topographically Complex Samples
- Electronic Reconstruction
Design of FCab and Confocal Microscopy
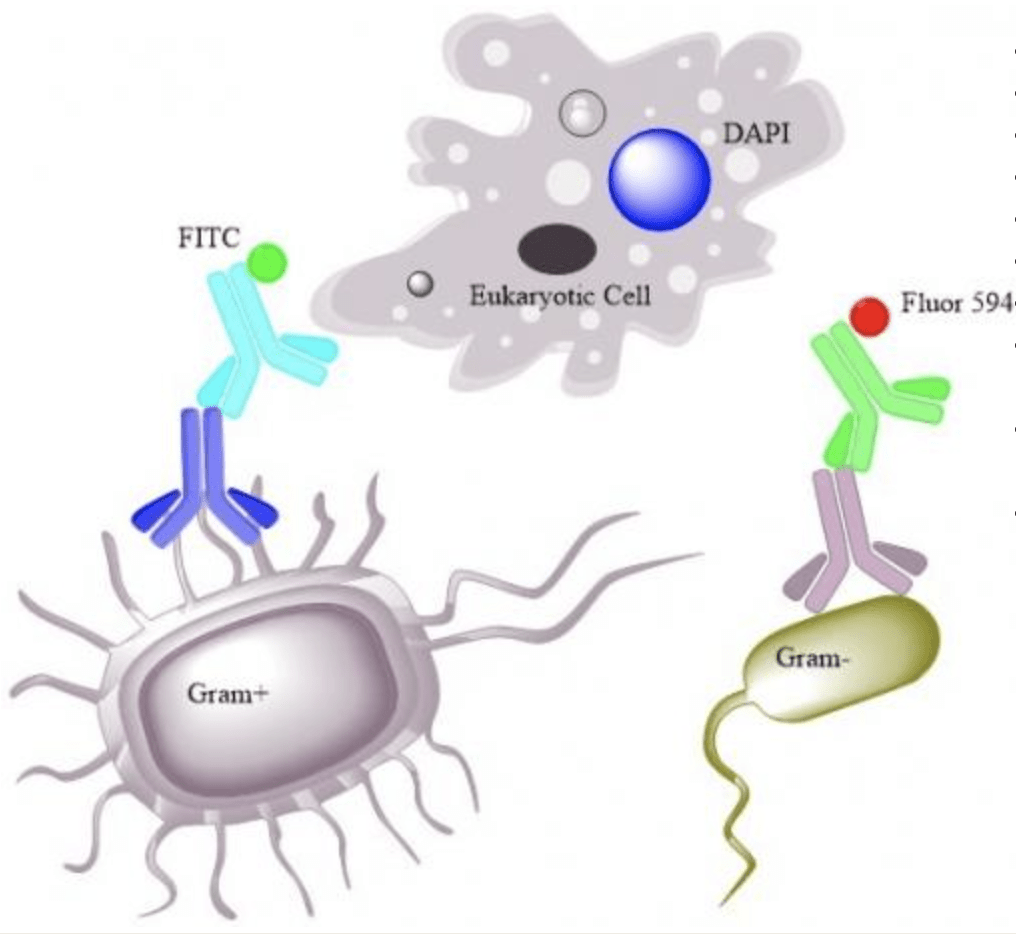
- Gram Positive
- Fluorescein Isothiocyanate (FITC)
- Ex 492nm-Em 518nm (Green)
- Gram Negative
- Alexa Fluor 594
- Ex 590nm-Em 617nm (Red)
- Eukaryotic Tissue
- 4’, 6-Diamidino-2-Phenindole, Dihydrochloride (DAPI)
- Binds to AT-rich regions of DNA (Nuclear stain)
- Ex 358nm-Em 461nm (Blue)