STUDY 1 (PANNEXIN)
This study focuses on the investigation of pannexin 1 channels as a novel emerging therapeutic target for the development of new pharmacotherapies in addictive disorders. This study stemmed from a preclinical work conducted in collaboration with Scripps Research Institute (Alcohol and Alcohol, 2019) and proof-of-concept RCT with AUD patients (ACER, 2024). Funding: R21 AA027614 (PI: Haass-Koffler) Clinical trial: NCT04218357
STUDY 2 (NORADRENALINE)
 This study focuses on the identification of endophenotypes that guide the application of personalized approaches (precision medicine) in the pharmacological treatment of AUD in order to better understand responses (or lack thereof) to pharmacological treatment. This study stemmed from a proof-of-concept RCT with AUD patients in which we demonstrated that blood pressure (clinical marker) (Drug Alcohol Depend, 2017, NEJM Journal Watch 2017) and family history of AUD (genetic marker) (Addict Biol, 2016) are independent moderators of doxazosin’s (α-1 inverse agonist) effect on alcohol consumption. Funding: R01 AA027760 (PI: Haass-Koffler) Clinical trial: NCT04135846
This study focuses on the identification of endophenotypes that guide the application of personalized approaches (precision medicine) in the pharmacological treatment of AUD in order to better understand responses (or lack thereof) to pharmacological treatment. This study stemmed from a proof-of-concept RCT with AUD patients in which we demonstrated that blood pressure (clinical marker) (Drug Alcohol Depend, 2017, NEJM Journal Watch 2017) and family history of AUD (genetic marker) (Addict Biol, 2016) are independent moderators of doxazosin’s (α-1 inverse agonist) effect on alcohol consumption. Funding: R01 AA027760 (PI: Haass-Koffler) Clinical trial: NCT04135846
STUDY 3 (CRFBP)
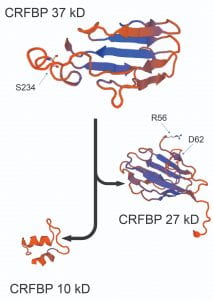 This is an innovative in vitro chimeric work that, in combination with human genetic variations, has discovered the role of corticotropin releasing factor binding protein (CRFBP) in alcohol use disorder (AUD) (Translational Psychiatry, 2016; Alcohol, 2018; SLAS, 2022). We transferred this chimeric biological assay to a robotic facility at Sanford Burnham Prebys Medical Discovery Institute. We screened >350,000 compounds, selected 2 allosteric modulators and tested them in an ex vivo preclinical alcohol model (electrophysiology) and in vivo behavioral pharmacology. Funding: R01 AA026589 and R01 AA0030888 (PI: Shefler; Subaward: Haass-Koffler)
This is an innovative in vitro chimeric work that, in combination with human genetic variations, has discovered the role of corticotropin releasing factor binding protein (CRFBP) in alcohol use disorder (AUD) (Translational Psychiatry, 2016; Alcohol, 2018; SLAS, 2022). We transferred this chimeric biological assay to a robotic facility at Sanford Burnham Prebys Medical Discovery Institute. We screened >350,000 compounds, selected 2 allosteric modulators and tested them in an ex vivo preclinical alcohol model (electrophysiology) and in vivo behavioral pharmacology. Funding: R01 AA026589 and R01 AA0030888 (PI: Shefler; Subaward: Haass-Koffler)
STUDY 4 (MDMA for PTSD COMORBIDITY)
MDMA-Assisted Therapy for Military veterans with PTSD and Alcohol Use Disorder. This is a study evaluating the safety and preliminary effectiveness of MDMA-assisted therapy for the treatment of co-occurring alcohol use disorder (AUD) and posttraumatic stress disorder (PTSD) in US veterans. We are using a longitudinal design to conduct an open label pilot trial of MDMA-AT with veterans who are seeking treatment for AUD-PTSD. The study is also collecting neuroimaging and biomarker data to examine brain changes pre-post treatment. Funding: Office of the Vice President Research (OVPR) and by the Center for Addiction and Disease Risk Exacerbation (CADRE), a NIH Center of Biomedical Research Excellence (COBRE) (MPI: Capone, Eaton, Haass-Koffler). Clinical Trial: NCT05943665
STUDY 5 (NEUROTHERAPIUTICS)
The goal of this Randomized Controlled Trial (RCT) application is to test the efficacy of intranasal insulin as a potential therapeutic for AUD. This study proposes a proof-of-concept, within-subject, crossover, double-blind, placebo-controlled human laboratory study with IN insulin compared to placebo in individuals with AUD. This study is in collaboration with HealthPartners (Drs. Bill Fray and Bhavani Kashyap). Funding: R34 AA031589 and Research Excellence Award (PI: Haass-Koffler) Clinical trial: NCT05988632.
