I. Photoelectron Spectroscopy of Size Selected Nanoclusters (AP1)
3. Gold Nanoclusters and Bimetallic Gold Nanoalloy Clusters
II. Electrospray Photoelectron Spectroscopy (AP2)
III. High-Resolution Photoelectron Imaging of Size-Selected Clusters (AP4)
IV. Exploratory Bulk Syntheses of Gold and Boron Nanoclusters and Materials
I. Photoelectron Spectroscopy of Size Selected Nanoclusters (AP1)
Current Research Projects:
I.1. Structural Evolution and Chemical Bonding of Size-Selected Boron Clusters: From Planar Structures to Borophenes and Borospherenes
Elemental boron is electron-deficient and boron compounds form interesting delocalized chemical bonds. Atomic clusters of boron provide an excellent platform to discover new structures and novel chemical bonds. The goal of this project is to elucidate the structural evolution and chemical bonding properties of boron clusters as a function of size and lay the foundation for new forms of boron nanostructures.
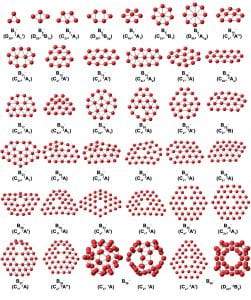
We have combined photoelectron spectroscopy of size-selected boron clusters with computational chemistry by our theoretical collaborators to show that small boron clusters possess planar structures, in contrast to that of bulk boron, which is dominated by three-dimensional polyhedral building blocks. The propensity for planarity has been found to be a result of both σ and π electron delocalization over the molecular plane, giving rise to concepts of σ and π multiple aromaticity. Because of its electron deficiency, boron cannot form graphene-like structures with a honeycomb hexagonal framework. We have found that the B36 cluster has a highly stable quasi-planar structure with a central hexagonal vacancy, providing the first experimental evidence that single-atom layer boron-sheets with hexagonal vacancies are viable. We coined the name “borophene” for the monolayer boron sheet. Borophenes have since been synthesized and characterized on inert substrates. In the photoelectron spectra of B40–, we observed two isomers, a planar structure and a three-dimensional cage structure. Computational studies revealed that the neutral B40 cage is extremely stable with a large HOMO-LUMO gap, representing the first all-boron fullerene, aka, borospherene.
A summary of the minimum energy structures, their point group symmetries, and electronic states of Bn– clusters (n = 3-30 and 35-38) resulting from joint photoelectron spectroscopy and theoretical studies
Selected recent publications:
“Planar Hexagonal B36 as a Potential Basis for Extended Single-Atom Layer Boron Sheets” (Z. A. Piazza, H. S. Hu, W. L. Li, Y. F. Zhao, J. Li, and L. S. Wang), Nature Commun. 5, 3113 (2014) .
“The B35 Cluster with a Double-Hexagonal Vacancy: A New and More Flexible Structural Motif for Borophenes” (W. L. Li, Q. Chen, W. J. Tian, H. Bai, Y. F. Zhao, H. S. Hu, J. Li, H. J. Zhai, S. D. Li, and L. S. Wang), J. Am. Chem. Soc. 136, 12257-12260 (2014) .
“Observation of an All-Boron Fullerene” (H. J. Zhai, Y. F. Zhao, W. L. Li, Q. Chen, H. Bai, H. S. Hu, Z. A. Piazza, W. J. Tian, H. G. Lu, Y. B. Wu, Y. W. Mu, G. F. Wei, Z. P. Liu, J. Li, S. D. Li, and L. S. Wang), Nature Chem. 6, 727-731 (2014) .
“Experimental and Theoretical Evidence of an Axially Chiral Borospherene” (Q. Chen, W. L. Li, Y. F. Zhao, S. Y. Zhang, H. S. Hu, H. Bai, H. R. Li, W. J. Tian, H. G. Lu, H. J. Zhai, S. D. Li, J. Li, and L. S. Wang), ACS Nano 9, 754-760 (2015) .
“Beyond Organic Chemistry: Aromaticity in Atomic Clusters” (A. I. Boldyrev and L. S. Wang), Phys. Chem. Chem. Phys. 18, 11589-11605 (2016) .
“Photoelectron Spectroscopy of Size-Selected Boron Clusters: From Planar Structures to Borophenes and Borospherenes” (L. S. Wang), Int. Rev. Phys. Chem. 35, 69-142 (2016) .
“B33– and B34–: Aromatic Planar Boron Clusters with A Hexagonal Vacancy” (Q. Chen, W. L. Li, X. Y. Zhao, H. R. Li, L. Y. Feng, H. J. Zhai, S. D. Li, and L. S. Wang), Eur. J. Inorg/ Chem. 4546-4551 (2017) .
“Recent Progress in Boron Clusters and Boron Materials (I): Borophene” (W. L. Li, H. S. Hu, Y. F. Zhao, X. Chen, T. T. Chen, T. Jian, L. S. Wang, and J. Li), Sci. Sin. Chim. 48, 1-10 (2018) .
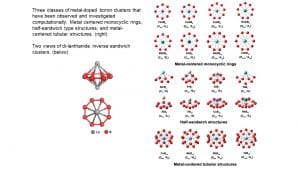
I.2. Metal Boride Clusters
Boron atoms possess strong chemical bonding capacities and can form boride materials with most metals, resulting in super-hard metal borides with transition metals and strong magnetic materials with lanthanide elements. Investigation of metal-doped boron clusters provides insights into the metal-boron bonds and may lead to the design of new metal boride materials. We have studied a variety of metal-doped boron clusters and found many novel structures and new types of chemical bonding, including metal-centered borometallic rings (M©Bn–), half-sandwich complexes, and metal-centered tubular structures. The discovery of the planar CoB18– cluster, in which the Co atom becomes an integral part of the planar boron structure has led to the possibility of metalloborophenes. Mono-lanthanide doped boron clusters tend to form half-sandwich structures, while di-lanthanide boron clusters have been found to form unprecedented inverse-sandwich structures.
Selected recent publications:
“Transition-Metal-Centered Monocyclic Boron Wheel Clusters (M©Bn): A New Class of Aromatic Borometallic Compounds” (C. Romanescu, T. R. Galeev, W. L. Li, A. I. Boldyrev, and L. S. Wang), Acc. Chem. Res.. 46, 350-358 (2013).
“Cobalt-Centered Boron Molecular Drums with the Highest Coordination Number in the CoB16– Cluster” (I. A. Popov, T. Jian, G. V. Lopez, A. I. Boldyrev, and L. S. Wang), Nature Commun.. 6, 8654 (2015).
“The Planar CoB18– Cluster as a Motif for Metallo-Borophenes” (W. L. Li, T. Jian, X. Chen, T. T. Chen, G. V. Lopez, J. Li, and L. S. Wang), Angew. Chem. Int. Ed.. 55, 7358-7363 (2016).
“Competition between Drum-like and Quasi-planar Structures in RhB18–: Motifs for Metallo-Boronanotubes or Metallo-Borophenes” (T. Jian, Wan-Lu Li, X. Chen, T. T. Chen, G. V. Lopez, J. Li, and L. S. Wang), Chem. Sci.. 7, 7020-7027 (2016).
“PrB7–: A Praseodymium-Doped Boron Cluster with a PrII Center Coordinated by a Doubly Aromatic Planar η7-B73- Ligand” (T. T. Chen, Wan-Lu Li, T. Jian, X. Chen, J. Li, and L. S. Wang), Angew. Chem. Int. Ed.. 56, 6916-6920 (2017).
“Bismuth-Boron Multiple Bonding in BiB2O– and Bi2B–” (T. Jian, L. F. Cheung, T. T. Chen, and L. S. Wang), Angew. Chem. Int. Ed.. 56, 9551-9555 (2017).
“From Planar Boron Clusters to Borophenes and Metalloborophenes” (Wan-Lu Li, X. Chen, T. Jian, T. T. Chen, J. Li, and L. S. Wang), Nat. Rev. Chem.. 1, 0071 (2017).
“Observation of Highly Stable and Symmetric Lanthanide Octa-Boron Inverse Sandwich Clusters” (Wan-Lu Li, T. T. Chen, D. H. Xing, X. Chen, J. Li, and L. S. Wang), Proc. Natl. Acad. Sci.(USA). 115, E6972-E6977 (2018).
I.3. Gold Nanoclusters and Bimetallic Gold Nanoalloy Clusters
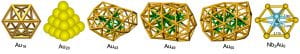
Gold is known to be the most inert metal, but small gold nanoparticles have been found to exhibit remarkable catalytic properties, a distinct nano-effect. A prerequisite to elucidate the mechanisms of the catalytic effects of nanogold is to understand the structures and chemical reactivity of gold clusters as a function of size. Small gold cluster anions are known to be planar up to 12 atoms. We have found that Au20 forms a beautiful tetrahedral structure. We have also discovered that the Au16 and Au17 clusters are hollow cages, similar to the carbon cage clusters. We are also interested in bimetallic gold clusters, which may exhibit new electronic, chemical, and structural properties relative to bare gold clusters.
Selected recent publications:
“Au20: A Tetrahedral Cluster” (J. Li, X. Li, H. J. Zhai, and L. S. Wang), Science. 299, 864-867 (2003).
“Probing the 2D to 3D Structural Transition in Gold Cluster Anions Using Argon Tagging” (W. Huang and L. S. Wang), Phys. Rev. Lett. 102, 153401 (2009).
“Probing the Structural Evolution of Medium-Sized Gold Clusters: Aun− (n = 27 to 35)” (N. Shao, W. Huang, Y. Gao, L. M. Wang, X. Li, L. S. Wang, and X. C. Zeng), J. Am. Chem. Soc. 132, 6596-6605 (2010).
“Structural Evolution of Core-Shell Gold Nanoclusters: Aun− (n = 42−50)” (S. Pande, W. Huang, N. Shao, L. M. Wang, N. Khetrapal, W. N. Mei, J. Tian, L. S. Wang, and X. C. Zeng), ACS Nano 10, 10013-10022 (2016).
“Probing the Structures of Gold-Aluminum Alloy Clusters AuxAly–: A Joint Experimental and Theoretical Study” (N. S. Khetrapal, J. Tian, R. Pal, G. V. Lopez, S. Pande, L. S. Wang, X. C. Zeng), Nanoscale 8, 9805-9814 (2016).
“Probing the Structural Evolution of Gold-Aluminium Bimetallic Clusters (Au2Aln–, n = 3−11) Using Photoelectron Spectroscopy and Theoretical Calculations” (N. S. Khetrapal, T. Jian, G. V. Lopez, S. Pande, L. S. Wang, and X. C. Zeng), J. Phys. Chem. C 121, 18234-18243 (2017).
“Nb2©Au6: A Molecular Wheel with a Short Nb-Nb Triple Bond Coordinated by an Au6 Ring and Reinforced by σ Aromaticity” (T. Jian, L. F. Cheung, J. Czekner, T. T. Chen, G. V. Lopez, W. L. Li, and L. S. Wang), Chem. Sci. 8, 7528-7536 (2017).
“Structural Evolution of Gold-Doped Bismuth Clusters AuBin– (n = 4−8)” (S. Pande, T. Jian, N. S. Khetrapal, L. S. Wang, and X. C. Zeng), J. Phys. Chem. C 122, 6947-6954 (2018).
“Determination of CO Adsorption Sites on Gold Clusters Aun– (n = 21-25) − A Size Region That Bridges the Pyramidal and Core-Shell Structures” (N. S. Khetrapal, L. S. Wang, and X. C. Zeng), J. Phys. Chem. Lett. 9, 5430-5439 (2018).
“Probing the Structure and Chemical Bonding of Auro-polyynes, Au(CC)nAu– (n = 1−3), Using High-Resolution Photoelectron Spectroscopy” (I. León, F. Ruipérez, J. M. Ugalde, and L. S. Wang), J. Chem. Phys. 149, 144307 (2018).
II. Electrospray Photoelectron Spectroscopy (AP2)
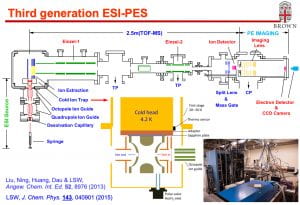
The Wang lab first introduced the technique of electrospray ionization (ESI) into spectroscopy to probe the properties of free multiply-charged anions [1-3], solution-phase chemistry in the gas phase [4,5], and the electronic structure of biologically-relevant molecules [6,7]. A second generation ESI-PES (photoelectron spectroscopy) apparatus was developed (AP3), in which we combined a cryogenically-cooled Paul trap with an ESI source for the first time [8,9]. Cold anions give rise to better PES resolution by eliminating vibrational hot bands [10] and H2-solvated anions in the cold ion trap led to the possibility of action spectroscopy [11]. At Brown University, we have developed the third generation ESI-PES apparatus, in which we combined a cryogenically-cooled Paul trap with high-resolution photoelectron imaging [12].
References:
[1]. “Probing the Potential Barriers and Intramolecular Electrostatic Interactions in Free Doubly Charged Anions” (L. S. Wang, C. F. Ding, X. B. Wang, and J. B. Nicholas), Phys. Rev. Lett. 81, 2667-2670 (1998).
[2]. “Observation of Negative Electron-Binding Energy in a Molecule” (X. B. Wang and L. S. Wang), Nature 400, 245-248 (1999).
[3]. “Photodetachment Photoelectron Spectroscopy of Multiply Charged Anions Using Electrospray Ionization” (L. S. Wang, C. F. Ding, X. B. Wang, and S. E. Barlow), Rev. Sci. Instrum. 70, 1957-1960 (1999).
[4]. “Bulk-Like Features in the Photoemission Spectra of Hydrated Doubly-Charged Anion Clusters” (X. B. Wang, X. Yang, J. B. Nicholas, and L. S. Wang), Science 294, 1322-1325 (2001).
[5]. “Probing Solution Phase Species and Chemistry in the Gas Phase” (X. B. Wang, X. Yang, and L. S. Wang), Int. Rev. Phys. Chem. 21, 472-498 (2002).
[6]. “Probing the Electronic Structure of [MoOS4]– Centers Using Anionic Photoelectron Spectroscopy” (X. B. Wang, F. E. Inscore, X. Yang, J. J. A. Cooney, J. H. Enemark, and L. S. Wang), J. Am. Chem. Soc. 124, 10182-10191 (2002).
[7]. “Probing the Intrinsic Electronic Structure of the Cubane [4Fe-4S] Cluster: Nature’s Favorite Cluster for Electron Transfer and Storage” ((X. B. Wang, S. Niu, X. Yang, S. K. Ibrahim, C. J. Pickett, T. Ichiye, and L. S. Wang), J. Am. Chem. Soc. 125, 14072-14081 (2003).
[8]. “Observation of Weak C-H…O Hydrogen-Bonding by Unactivated Alkanes” (X. B. Wang, H. K. Woo, B. Kiran, and L. S. Wang), Angew. Chem. Int. Ed. 44, 4968-4972 (2005).
[9]. “Development of a Low-Temperature Photoelectron Spectroscopy Instrument Using an Electrospray Ion Source and a Cryogenically Controlled Ion Trap” (X. B. Wang and L. S. Wang), Rev. Sci. Instrum. 79, 073108 (2008).
[10]. “Vibrational Cooling in a Cold Ion Trap: Vibrationally Resolved Photoelectron Spectroscopy of Cold C60– Anions” (X. B. Wang, H. K. Woo, and L. S. Wang), J. Chem. Phys. 123, 051106 (2005).
[11]. “Observation of H2 Aggregation onto a Doubly Charged Anion in a Temperature-Controlled Ion Trap” (X. B. Wang, X. P. Xing, and L. S. Wang), J. Phys. Chem. A 112, 13271-13274 (2008).
[12]. “Electrospray Photoelectron Spectroscopy: From Multiply-Charged Anions to Ultracold Anions” (L. S. Wang), J. Chem. Phys. 143, 040901 (2015). (Invited perspective)
Current Research Projects:
The third generation ESI-PES apparatus can reach an energy resolution of 1~2 cm-1 for low energy electrons, which represents an improvement of about two orders of magnitude over the magnetic-bottle apparatus. This tremendous enhancement in electron energy resolution in combination with the cryogenic cooling of trapped anions from ESI has greatly expanded our capability to investigate the electronic structure and vibrational spectroscopy of solution anions and the resulting radical species upon electron detachment. We are interested in inorganic, organic, and biologically relevant anions, in particular, polycyclic aromatic molecules and their derivatives, which are of both environmental and astrochemical interests.
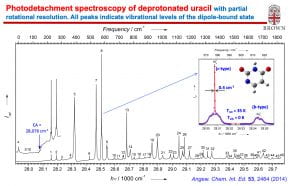
With a tunable dye laser, we are also able to conduct photodetachment spectroscopy (PDS) by scanning the photon energy while monitoring the total electron yield. PDS allows us to probe electronic excited states of anions and to perform resonant two-photon PES and PDS. Dipole-bound excited states of anions with polar neutral cores are an interesting class of excited states for anions, analogous to Rydberg states of neutral molecules. These excited states are just below the electron detachment thresholds with binding energies on the order of a few to few hundred cm-1, meaning that any vibrational excitation in the dipole-bound states can undergo vibrationally-induced autodetachment. The cold anions make it possible to measure PDS via vibrational autodetachment and obtain well-resoled vibronic transitions with optical resolution in the PDS. By tuning the detachment laser to specific vibronic levels of the dipole-bound excited state, we can obtain resonantly enhanced PES (rPES). We have shown that rPES is highly non-Franck-Condon due to the propensity rule of the vibrational autodetachment and can yield much more vibrational information than ordinary non-resonant PES. Franck-Condon-inactive and even symmetry-forbidden vibrational modes are routinely observed, resulting in rich vibrational information for the neutral final states. We are interested in using PDS and rPES not only to obtain more detailed and accurate spectroscopic information about the neutral radicals upon electron detachment, but also to probe the dynamics of the vibronic coupling during the vibrationally-induced electron autodetachment, which involves the transfer of one quantum of vibrational energy (Δv = –1 propensity rule) in the dipole-bound state to the outgoing electron.
Selected recent publications:
“Observation of Mode-Specific Vibrational Autodetachment from Dipole-Bound States of Cold Anions”, (H. T. Liu, C. G. Ning, D. L. Huang, P. D. Dau, and L. S. Wang), Angew. Chem. Int. Ed. 52, 8976 (2013).
“High-Resolution Photoelectron Imaging of Cold C60− Anions and Accurate Determination of the Electron Affinity of C60” (D. L. Huang, P. D. Dau, H. T. Liu, and L. S. Wang), J. Chem. Phys. 140, 224315 (2014).
“Vibrational State-Selective Resonant Two-Photon Photoelectron Spectroscopy of AuS– via a Spin-Forbidden Excited State” (H. T. Liu, D. L. Huang, Y. Liu, L. F. Cheung, P. D. Dau, C. G. Ning, and L. S. Wang), J. Chem. Phys. 6, 637-642 (2015).
“Probing the Vibrational Spectroscopy of the Deprotonated Thymine Radical by Photodetachment and State-Selective Autodetachment Photoelectron Spectroscopy via Dipole-Bound States” (D. L. Huang, H. T. Liu, C. G. Ning, G. Z. Zhu, and L. S. Wang), Chem. Sci. 6, 3129-3138 (2017).
“Resonant Photoelectron Imaging of Deprotonated Uracil Anion via Vibrational Levels of a Dipole-Bound Excited State” (D. L. Huang, H. T. Liu, C. G. Ning, P. D. Dau, and L. S. Wang), Chem. Phys.. 482, 374-383 (2017).
“Conformation-Selective Resonant Photoelectron Imaging from Dipole-Bound States of Cold 3-Hydroxyphenoxide” (G. Z. Zhu, D. H. Huang, and L. S. Wang), J. Chem. Phys. 147, 013910 (2017).
“Observation of Excited Quadrupole-Bound States in Cold Anions” (G. Z. Zhu, Y. Liu, and L. S. Wang), Phys. Rev. Lett. 119, 023002 (2017).
“Probing the Interaction between the Encapsulated Water Molecule and the Fullerene Cages in H2O@C60– and H2O@C59N–” (G. Z. Zhu, Y. Liu, Y. Hashikawa, Q. F. Zhang, Y. Murata, and L. S. Wang), Chem. Sci. 9, 5666-5671 (2018).
III. High-Resolution Photoelectron Imaging of Size-Selected Clusters (AP4)
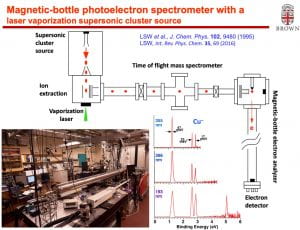
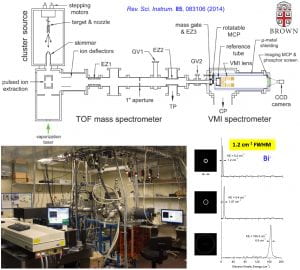
We have developed a new velocity-map imaging apparatus equipped with a laser-vaporization supersonic cluster source and a time-of-flight mass spectrometer for high-resolution photoelectron spectroscopy studies of size-selected cluster anions. Vibrationally cold anionic clusters are produced using a laser-vaporization supersonic source, size-selected by a time-of-flight mass spectrometer, and then focused co-linearly into the interaction zone of the high-resolution velocity-map imaging (VMI) system. The multi-lens VMI design was optimized via systematic SIMION simulations to achieve superior focusing power over a large energy range. The new VMI system can reach a resolution of 1.2 cm-1 (FWHM) for near threshold electrons while maintaining photoelectron kinetic energy resolutions (ΔKE/KE) of ~0.53% for high energy electrons.
Current Research Projects:
The high-resolution capability of this apparatus allows vibrational information to be obtained for small clusters, which rivals infrared spectroscopy and is highly valuable for structural determination. One of the first experiments we performed was on the Au4– cluster, for which we were able to resolve vibrational structures for three modes, including a mode with a low frequency of 17 cm-1. The vibrational information unequivocally confirmed the Y-shaped C2v structure for Au4–. One of our major current focus is on boron and boride clusters. We have completely resolved the vibrational structures for the B11 and B12 clusters and confirmed their planar structures. On-going research involves high-resolution studies of metal-doped boron clusters. We have also observed a dipole-bound excited state for the C2P– cluster and obtained interesting information about the weak spin-coupling of the dipole-bound electron and the electrons in the neutral core via resonant photoelectron spectroscopy. We are interested in a wide range of cluster and molecular systems, in particular, those of astrochemical interests. We are also interested in probing dipole-bound excited states of polar anionic clusters, which allow both photodetachment spectroscopy and resonant photoelectron spectroscopy to be conducted to yield rich vibrational information for the underlying neutral clusters.
Selected recent publications:
“High Resolution Photoelectron Imaging of Au2–”, (I. León, Z. Yang, and L. S. Wang), J. Phys. Chem. 138, 184304 (2013).
“Vibrational Spectroscopy of Au4 from High Resolution Photoelectron Imaging”, (Z. Yang, I. León, and L. S. Wang), J. Chem. Phys. 139, 021106 (2013).
“The Design and Construction of a High-Resolution Velocity-Map Imaging Apparatus for Photoelectron Spectroscopy Studies of Size-Selected Clusters”, (I. León, Z. Yang, H. T. Liu, and L. S. Wang), Rev. Sci. Instrum. 85, 083106 (2014).
“High Resolution Photoelectron Imaging of UO– and UO2– and the Low-Lying Electronic States and Vibrational Frequencies of UO and UO2”, (J. Czekner, G. V. Lopez, and L. S. Wang), J. Chem. Phys. 141, 244302 (2014).
“Probing the Electronic Structure and Au–C Chemical Bonding in AuCn– and AuCnH– (n = 2, 4, 6) Using High-Resolution Photoelectron Spectroscopy”, (I. León, F. Ruipérez, J. Ugalde, and L. S. Wang), J. Chem. Phys. 145, 064304 (2016).
“Probing the Structures of Neutral B11 and B12 Using High Resolution Photoelectron Imaging of B11– and B12–”, (J. Czekner, L. F. Cheung, and L. S. Wang), J. Phys. Chem. C 121, 10752-10759 (2017).
“A High-Resolution Photoelectron Imaging and Theoretical Study of CP‾ and C2P‾”, (J. Czekner, L. F. Cheung, E. L. Johnson, R. C. Fortenberry, and L. S. Wang), J. Chem. Phys. 148, 044301 (2018).
“Probing the Coupling of a Dipole-Bound Electron with the Molecular Core”, (J. Czekner, L. F. Cheung, G. S. Kocheril, and L. S. Wang), Chem. Sci. 10, (2019) DOI: 10.1039/c8sc04771e.
“High Resolution Photoelectron Imaging of Boron-Bismuth Binary Clusters: Bi2Bn– (n = 2–4)”, (L. F. Cheung, J. Czekner, G. S. Kocheril, and L. S. Wang), submitted
IV. Exploratory Bulk Sytheses of Gold and Boron Nanoclusters and Materials
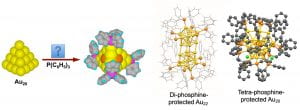
A longstanding objective of cluster science is to discover highly stable clusters and to use them as models for catalysts and building blocks for cluster-assembled materials. The discovery of catalytic properties of gold nanoparticles (AuNPs) has stimulated wide interests in gaseous size-selected gold clusters. Ligand-protected AuNPs have also been extensively investigated to understand their size-dependent catalytic and optical properties. However, the need to remove ligands can introduce uncertainties both in the structures and sizes of ligand-protected AuNPs for catalytic applications. Ideal model catalysts should be atomically-precise AuNPs with well-defined structures and uncoordinated surface sites as in-situ active centers. The tetrahedral (Td) Au20 pyramidal cluster, discovered in the Wang lab to be highly stable in the gas phase, provided a unique opportunity for such an ideal model system. The Td-Au20 consists of four Au(111) faces with all its atoms on the surface. Bulk syntheses of Td-Au20 with appropriate ligands would allow its catalytic and optical properties to be investigated and harnessed. The different types of its surface atoms would allow site-specific chemistry to be exploited.
We hypothesized that if the four corner atoms of Td-Au20 were coordinated by ligands the cluster would still contain sixteen uncoordinated surface sites as potential in-situ catalytic active centers. We have been trying to use di-phosphine ligands [(Ph)2P(CH2)nP(Ph)2 or Ln] to synthesize the Td-Au20 cluster. When the L8 diphosphine ligand was used, a remarkable Au22 nanocluster with eight uncoordinated Au sites, Au22(L8)6, was synthesized instead. With a tetraphosphine-ligand (PP3), a new Au20 nanocluster, [Au20(PP3)4]Cl4, was isolated with high yield. The crystal structure of the new Au20 core did not have the expected Td structure, but rather an intrinsically chiral gold core. The surface of the new chiral-Au20 was fully coordinated and it was found to be highly stable chemically. The Au22(L8)6 nanocluster represents the first and only gold core with uncoordinated gold atoms, providing potentially eight in-situ catalytic active sites. The Au22 nanoclusters dispersed on oxide supports were found to catalyze CO oxidation and activate H2 without ligand removal. With further understanding about the formation mechanisms of gold nanoclusters in solution, it is conceivable that the Td-Au20 can be eventually synthesized, allowing its novel catalytic and optical properties to be explored. It is possible that a whole family of new atomically-precise gold nanoclusters can be created with different phosphine ligands.
With the discovery of many interesting boron and doped-boron clusters, we are also interested in exploratory syntheses of novel boron clusters and related borane compounds. We are interested in both laser-based methods and solution-based syntheses. We have established important national and international collaborations for the solution syntheses of novel boron cluster compounds.
Selected recent publications:
“Controlling Gold Nanoclusters by Diphosphine Ligands”, (J. Chen, Q. F. Zhang, T. A. Bonaccorso, P. G. Williard, and L. S. Wang), J. Am. Chem. Soc. 136, 92-95 (2014).
“Synthesis and Structure Determination of a New Au20 Nanocluster Protected by Tripodal Tetraphosphine Ligands”, (J. Chen, Q. F. Zhang, P. G. Williard, and L. S. Wang), Inorg. Chem. 53, 3932-3934 (2014).
“Polymorphism of Phosphine-Protected Gold Nanoclusters: Synthesis and Characterization of a New Au22(C28H28OP2)7 Cluster”, (Q. F. Zhang, P. G. Williard, and L. S. Wang), Small 12, 2518-2525 (2016).
“Second-Order Nonlinear Optical Scattering Properties of Phosphine-Protected Au20 Clusters”, (S. Knoppe, Q. F. Zhang, X. K. Wan, Q. M. Wang, L. S. Wang, and T. Verbiest), Ind. Eng. Chem. Res. 55, 10500-10506 (2016).
“Diphosphine-Protected Au22 Nanoclusters on Oxide Supports Are Active for Gas-Phase Catalysis Without Ligand Removal”, (Z. L. Wu, G. X. Hu, D. E. Jiang, D. R. Mullins, Q. F. Zhang, L. F. Allard Jr, L. S. Wang, and S. H. Overbury), Nano Lett. 16, 6560-6567 (2016).
““[(Cp2M)2B9H11] (M = Zr or Hf): Early Transition Metal ‘Guarded’ Heptaborane with Strong Covalent and Electrostatic Bonding”, (A. De, Q. F. Zhang, B. Mondal, L. F. Cheung, S. Kar, K. Saha, B. Varghese, L. S. Wang, and S. Ghosh), Chem. Sci. 9, 1975-1981 (2018).
“Elucidation of the Formation Mechanism of Octahydrotriborate Anion (B3H8−) through the Nucleophilicity of the B−H Bond”, (X. M. Chen, N. N. Ma, Q. F. Zhang, J. Wang, X. G. Feng, C. G. Wei, L. S. Wang, J. Zhang, and X. N. Chen), J. Am. Chem. Soc. 140, 6718-6726 (2018).
“Toward Solution Syntheses of the Tetrahedral Au20 Pyramid and Atomically-Precise Gold Nanoclusters with Uncoordinated Sites”, (Q. F. Zhang, X. N. Chen, and L. S. Wang), Acc. Chem. Res. 51, 2159-2168 (2018).