Schmitt, P.R., Dwyer, K.D., Minor, A.J., & Coulombe, K.L.K., 2022. Wet-Spun Polycaprolactone Scaffolds Provide Customizable Anisotropic Viscoelastic Mechanics for Engineered Cardiac Tissues. Polymers. https://doi.org/10.3390/polym14214571
Daley, M. C., Mende, U., Choi, B. R., McMullen, P. D., & Coulombe, K., 2022. Beyond pharmaceuticals: Fit-for-purpose new approach methodologies for environmental cardiotoxicity testing. ALTEX. https://doi.org/10.14573/altex.2108261
Schmitt, P.R., Dwyer, K.D., Coulombe, K.L.K., 2022. Current Applications of Polycaprolactone as a Scaffold Material for Heart Regeneration. ACS Appl Bio Mater. https://doi.org/10.1021/acsabm.2c00174
Minor, A.J., Coulombe, K.L.K., 2022. Stimulating Calcium Handling in hiPSC-Derived Engineered Cardiac Tissues Enhances Force Production. Stem Cells Translational Medicine 11, 97–106. https://doi.org/10.1093/stcltm/szab002
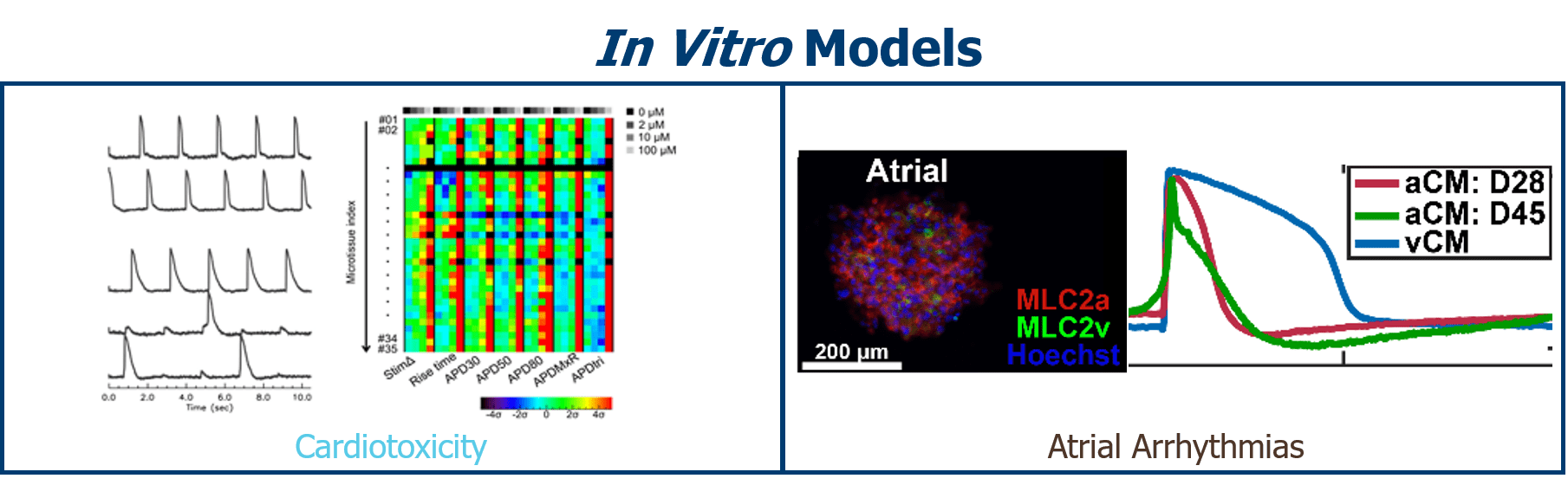
Soepriatna, A.H., Kim, T.Y., Daley, M.C., Song, E., Choi, B.-R., Coulombe, K.L.K., 2021. Human Atrial Cardiac Microtissues for Chamber-Specific Arrhythmic Risk Assessment. Cel. Mol. Bioeng. 14, 441–457. https://doi.org/10.1007/s12195-021-00703-x
Reid, J.A., Dwyer, K.D., Schmitt, P.R., Soepriatna, A.H., Coulombe, K., Callanan, A., 2021. Architected fibrous scaffolds for engineering anisotropic tissues. Biofabrication. https://doi.org/10.1088/1758-5090/ac0fc9
Bai, Y., Kaiser, N.J., Coulombe, K.L.K., Srivastava, V., 2021. A continuum model and simulations for large deformation of anisotropic fiber-matrix composites for cardiac tissue engineering. J Mech Behav Biomed Mater 121, 104627. https://doi.org/10.1016/j.jmbbm.2021.104627
Kofron, C.M., Kim, T.Y., Munarin, F., Soepriatna, A.H., Kant, R.J., Mende, U., Choi, B.-R., Coulombe, K.L.K., 2021. A predictive in vitro risk assessment platform for pro-arrhythmic toxicity using human 3D cardiac microtissues. Sci Rep 11, 10228. https://doi.org/10.1038/s41598-021-89478-9
Dwyer, K.D., Coulombe, K.L.K., 2021. Cardiac mechanostructure: Using mechanics and anisotropy as inspiration for developing epicardial therapies in treating myocardial infarction. Bioact Mater 6, 2198–2220. https://doi.org/10.1016/j.bioactmat.2020.12.015
Rountree, I., Polucha, C., Coulombe, K.L.K., Munarin, F., 2021. Assessing the Angiogenic Efficacy of Pleiotrophin Released from Injectable Heparin-Alginate Gels. Tissue Eng Part A. https://doi.org/10.1089/ten.TEA.2020.0335
Munarin, F., Kabelac, C., Coulombe, K.L.K., 2021. Heparin-modified alginate microspheres enhance neovessel formation in hiPSC-derived endothelial cells and heterocellular in vitro models by controlled release of vascular endothelial growth factor. J Biomed Mater Res A. https://doi.org/10.1002/jbm.a.37168
Kant, R.J., Bare, C.F., Coulombe, K.L.K., 2021. Tissues with patterned vessels or protein release induce vascular chemotaxis in an in vitro platform. Tissue Eng Part A. https://doi.org/10.1089/ten.TEA.2020.0269
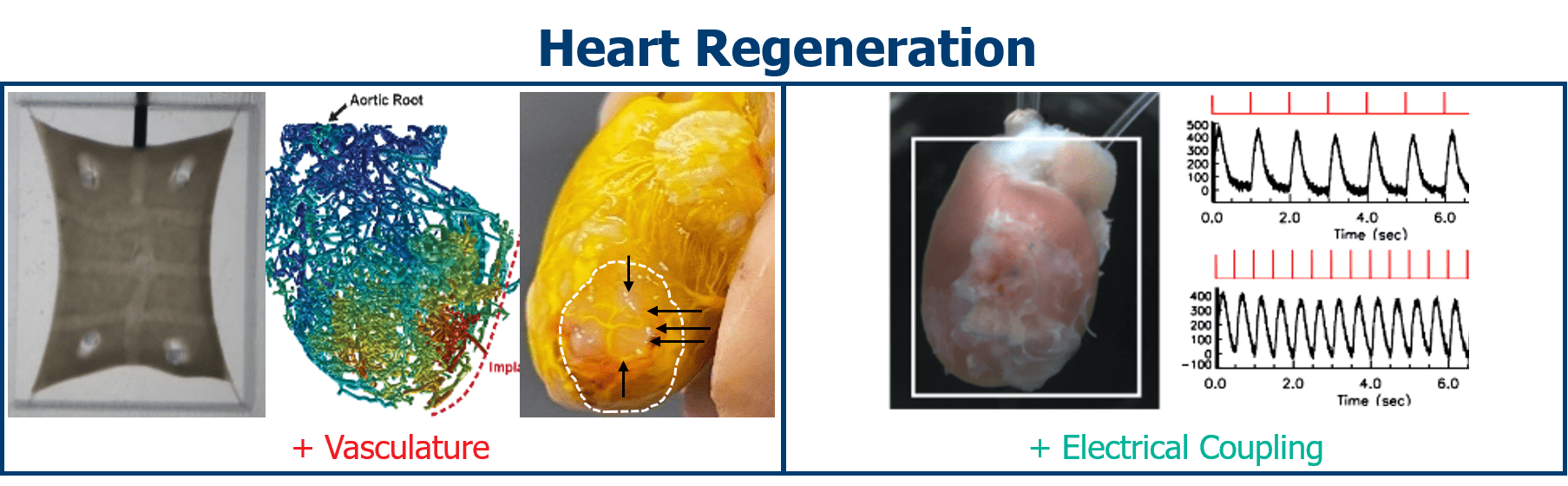
Munarin, F., Kant, R.J., Rupert, C.E., Khoo, A., Coulombe, K.L.K., 2020. Engineered human myocardium with local release of angiogenic proteins improves vascularization and cardiac function in injured rat hearts. Biomaterials 251, 120033. https://doi.org/10.1016/j.biomaterials.2020.120033
Minor, A.J., Coulombe, K.L.K., 2020. Engineering a collagen matrix for cell-instructive regenerative angiogenesis. J Biomed Mater Res B Appl Biomater. https://doi.org/10.1002/jbm.b.34573
Bloise, N., Rountree, I., Polucha, C., Montagna, G., Visai, L., Coulombe, K.L.K., Munarin, F., 2020. Engineering Immunomodulatory Biomaterials for Regenerating the Infarcted Myocardium. Front Bioeng Biotechnol 8, 292. https://doi.org/10.3389/fbioe.2020.00292
Rupert, C.E., Irofuala, C., Coulombe, K.L.K., 2020. Practical adoption of state-of-the-art hiPSC cardiomyocyte differentiation techniques. PLoS One 15, e0230001. https://doi.org/10.1371/journal.pone.0230001
Rupert, C.E., Kim, T.Y., Choi, B.-R., Coulombe, K.L.K., 2020. Human Cardiac Fibroblast Number and Activation State Modulate Electromechanical Function of hiPSC-Cardiomyocytes in Engineered Myocardium. Stem Cells Int 2020, 9363809. https://doi.org/10.1155/2020/9363809
Kaiser, N.J., Bellows, J.A., Kant, R.J., Coulombe, K.L.K., 2019. Digital Design and Automated Fabrication of Bespoke Collagen Microfiber Scaffolds. Tissue Eng Part C Methods 25, 687–700. https://doi.org/10.1089/ten.TEC.2018.0379
Kaiser, N.J., Kant, R.J., Minor, A.J., Coulombe, K.L.K., 2019. Optimizing Blended Collagen-Fibrin Hydrogels for Cardiac Tissue Engineering with Human iPSC-derived Cardiomyocytes. ACS Biomater Sci Eng 5, 887–899. https://doi.org/10.1021/acsbiomaterials.8b01112
Kaiser, N.J., Munarin, F., Coulombe, K.L.K., 2018. Custom Engineered Tissue Culture Molds from Laser etched Masters. J Vis Exp. https://doi.org/10.3791/57239
Kant, R.J., Coulombe, K.L.K., 2018. Integrated approaches to spatiotemporally directing angiogenesis in host and engineered tissues. Acta Biomater 69, 42–62. https://doi.org/10.1016/j.actbio.2018.01.017
Liu, M., Shi, G., Zhou, A., Rupert, C.E., Coulombe, K.L.K., Dudley, S.C., 2018. Activation of the unfolded protein response downregulates cardiac ion channels in human induced pluripotent stem cell-derived cardiomyocytes. J Mol Cell Cardiol 117, 62–71. https://doi.org/10.1016/j.yjmcc.2018.02.011
Munarin, F., Kaiser, N.J., Kim, T.Y., Choi, B.-R., Coulombe, K.L.K., 2017. Laser-Etched Designs for Molding Hydrogel-Based Engineered Tissues. Tissue Eng Part C Methods 23, 311–321. https://doi.org/10.1089/ten.TEC.2017.0068
Rupert, C.E., Coulombe, K.L.K., 2017. IGF1 and NRG1 Enhance Proliferation, Metabolic Maturity, and the Force-Frequency Response in hESC-Derived Engineered Cardiac Tissues. Stem Cells Int 2017, 7648409. https://doi.org/10.1155/2017/7648409
Rupert, C.E., Chang, H.H., Coulombe, K.L.K., 2017. Hypertrophy changes 3D shape of hiPSC cardiomyocytes: Implications for cellular maturation in regenerative medicine. Cell Mol Bioeng 10, 54–62. https://doi.org/10.1007/s12195-016-0462-7
Roberts, M.A., Tran, D., Coulombe, K.L.K., Razumova, M., Regnier, M., Murry, C.E., Zheng, Y., 2016. Stromal Cells in Dense Collagen Promote Cardiomyocyte and Microvascular Patterning in Engineered Human Heart Tissue. Tissue Eng Part A 22, 633–644. https://doi.org/10.1089/ten.TEA.2015.0482
Rupert, C.E., Coulombe, K.L., 2015. The roles of neuregulin-1 in cardiac development, homeostasis, and disease. Biomark Insights 10, 1–9. https://doi.org/10.4137/BMI.S20061
Kaiser, N.J., Coulombe, K.L.K., 2015. Physiologically inspired cardiac scaffolds for tailored in vivo function and heart regeneration. Biomed Mater 10, 034003. https://doi.org/10.1088/1748-6041/10/3/034003
Gerbin, K.A., Yang, X., Murry, C.E., Coulombe, K.L.K., 2015. Enhanced Electrical Integration of Engineered Human Myocardium via Intramyocardial versus Epicardial Delivery in Infarcted Rat Hearts. PLoS One 10, e0131446. https://doi.org/10.1371/journal.pone.0131446
Coulombe, K.L.K., Bajpai, V.K., Andreadis, S.T., Murry, C.E., 2014. Heart regeneration with engineered myocardial tissue. Annu Rev Biomed Eng 16, 1–28. https://doi.org/10.1146/annurev-bioeng-071812-152344
Coulombe, K.L.K., Murry, C.E., 2014. Vascular Perfusion of Implanted Human Engineered Cardiac Tissue. Proc IEEE Annu Northeast Bioeng Conf 2014. https://doi.org/10.1109/NEBEC.2014.6972763