Background
Propionibacterium acnes
- Slow-growing, anaerobic-aerotolerant, gram positive rod.
- Commonly found as part of the natural flora of the large intestine, conjunctiva, oral cavity, and in the pilosebaceous follicles of the skin.
- Most common organism colonizing shoulder area.
- Commonly recognized as the causative agent of acne vulgaris.
- Recognized as an opportunistic pathogen colonizing implants on the shoulder region.
- Most frequently isolated pathogen in prosthetic shoulder joint infections.
- Current pre-surgical dressings like chlorheximide gluconate appear to not effective against it.
Methodology
- Cultured in a humidified anaerobic environment in Reduced Clostridial Media (BD) for 48hrs at 37oC.
- Implants inoculated with 1×107 cfu/ml for variable times of adherence and proliferation.
- Dehydrated, Fixed, and Labeled.
- Visualized via SEM and CLSM.
- Coated implants dip-coated in 95% 10X (95% TiO2: 5% PDMS) and 100% Ag.
Experimental Conditions
| 4-20 | 8-16 | 12-12 | 16-8 | 20-4 |
| Control | Experimental | Control |
| Uncoated | 95% 10X | 100% Ag |
Results
Spinal Implant Materials
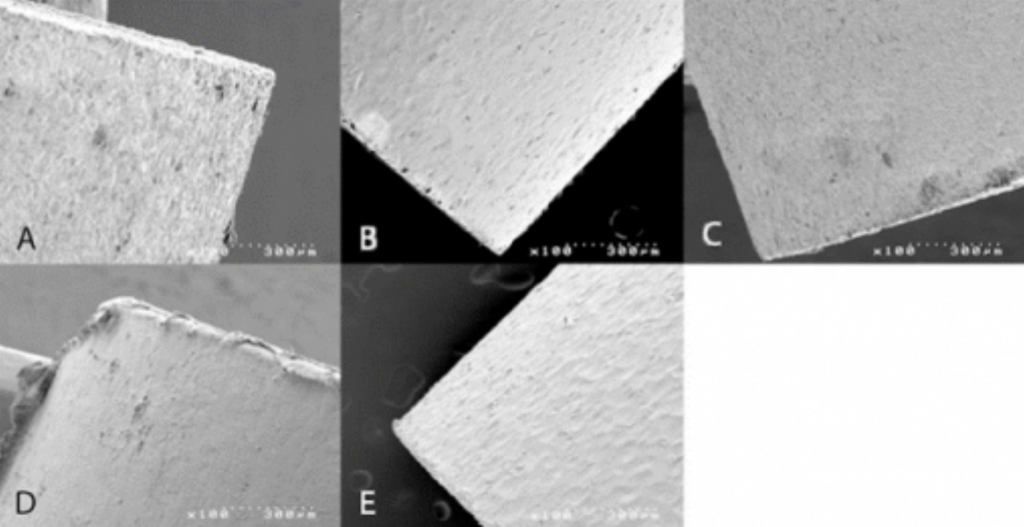
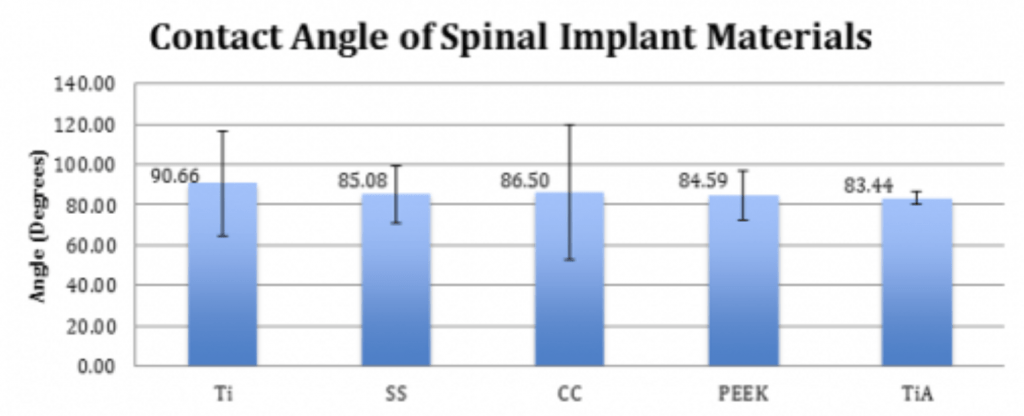


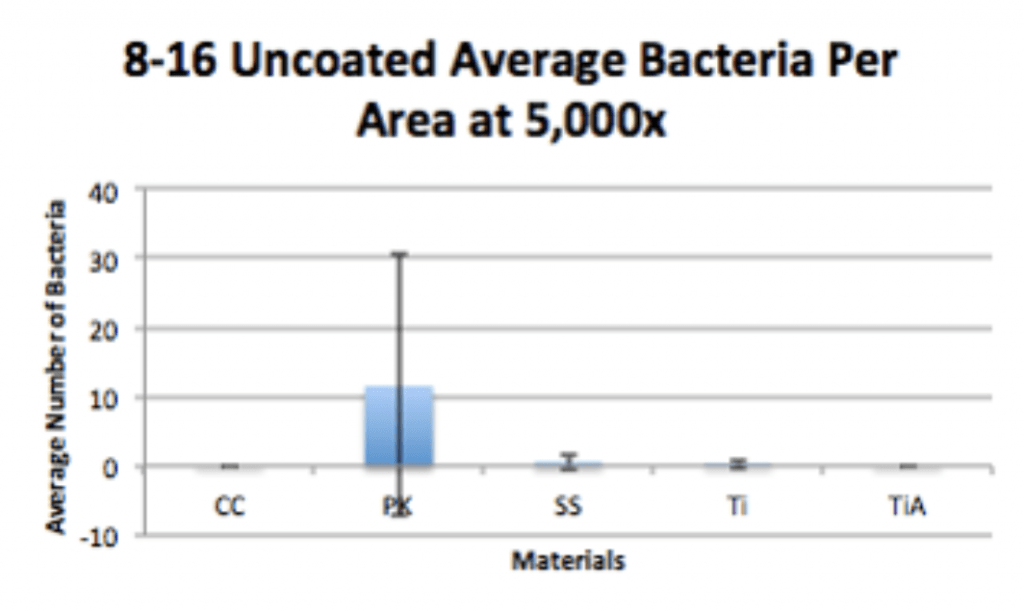
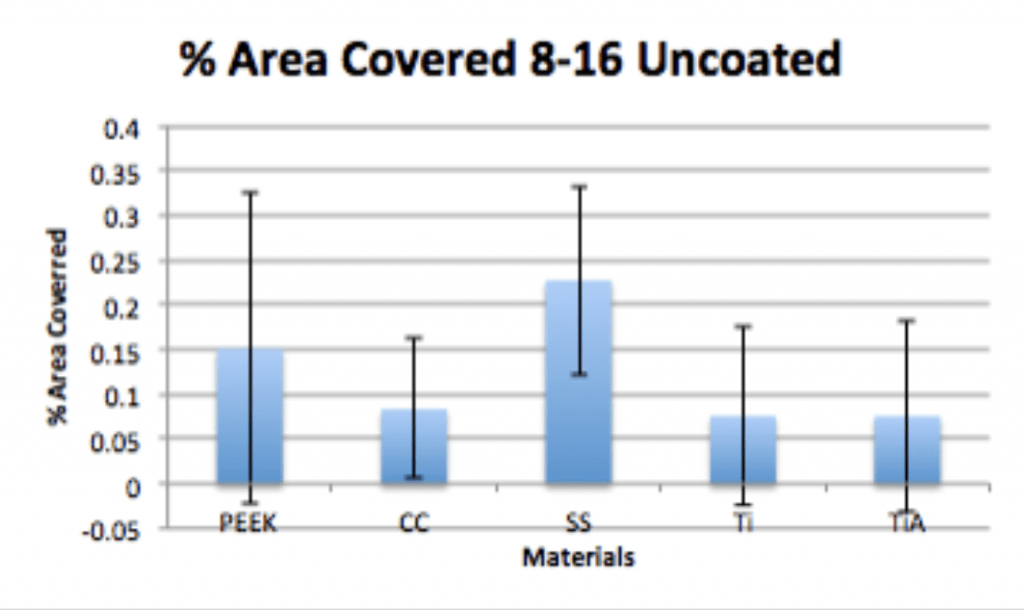
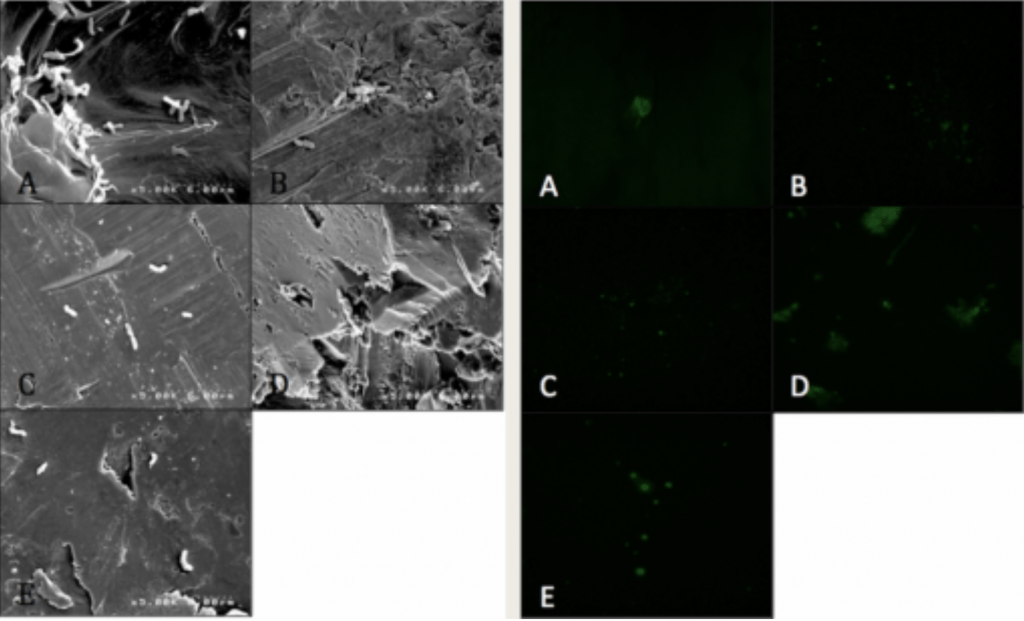

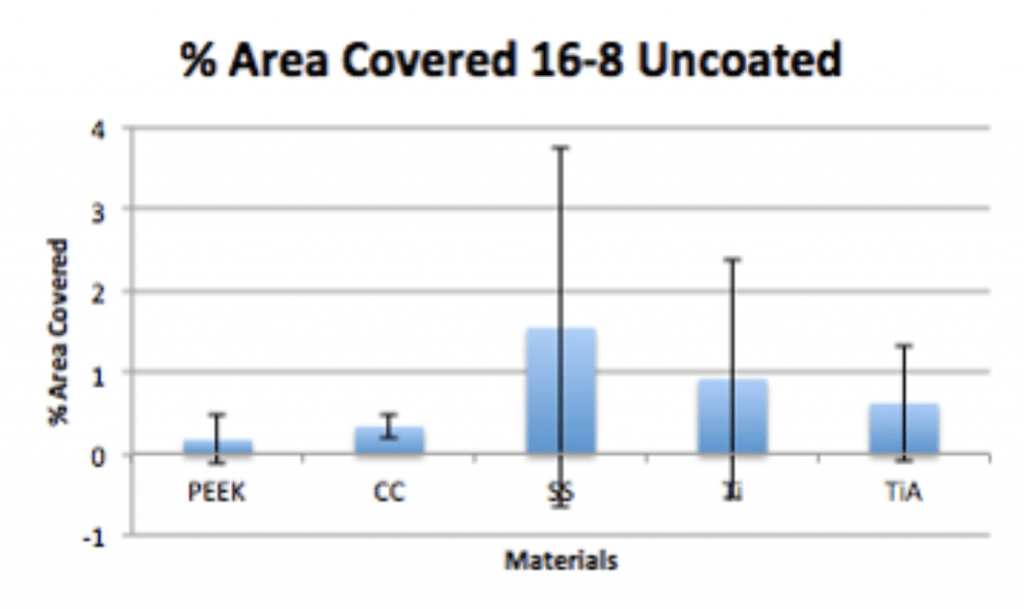
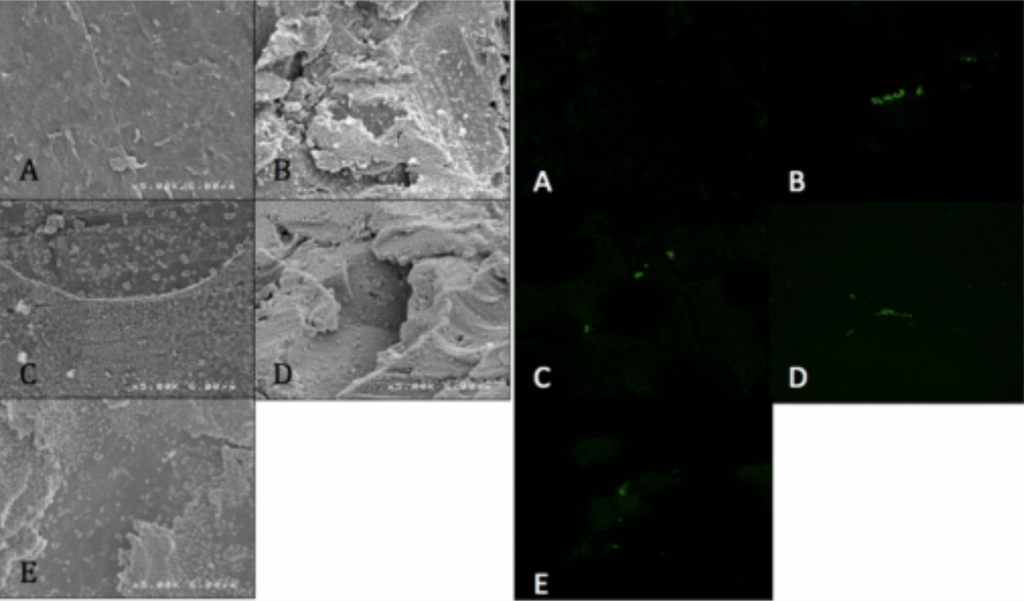
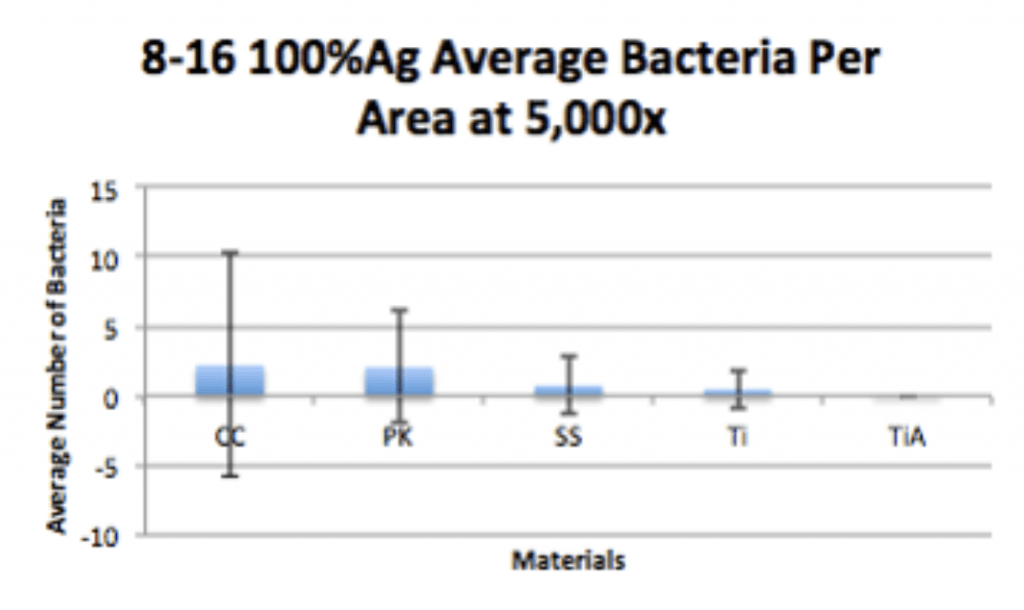
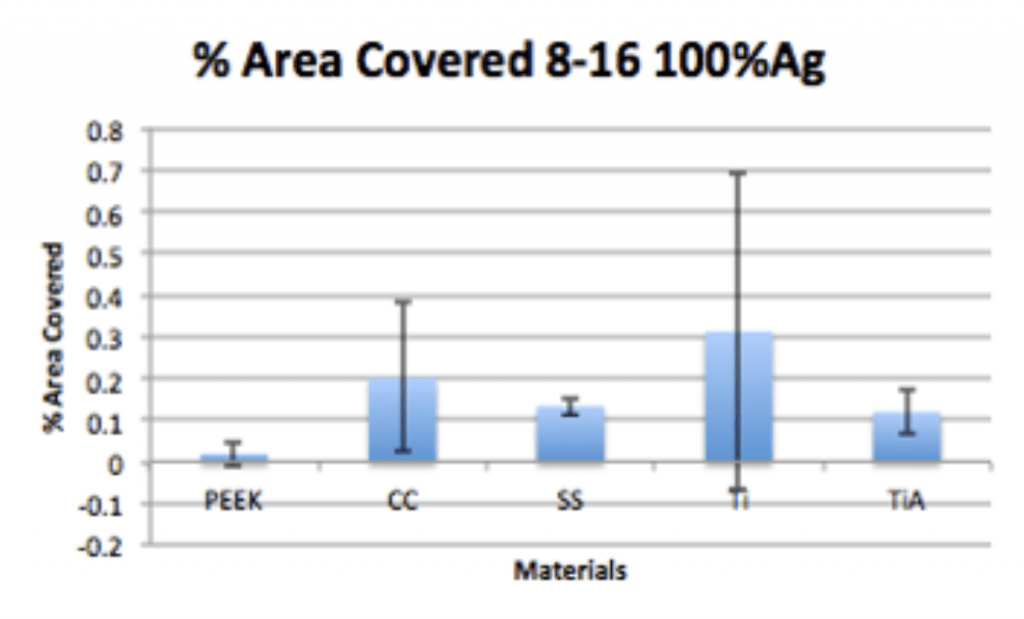
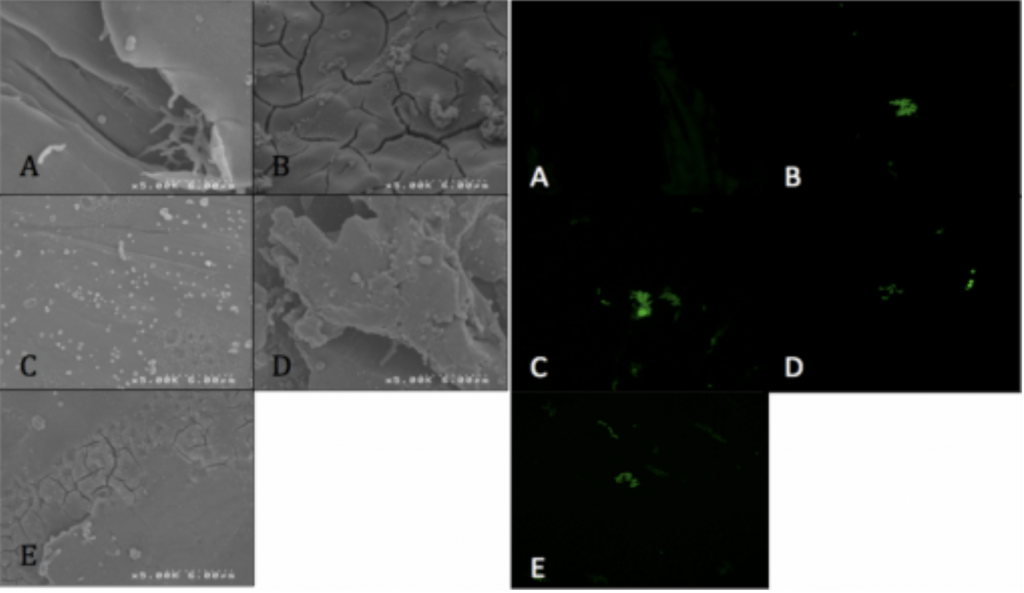
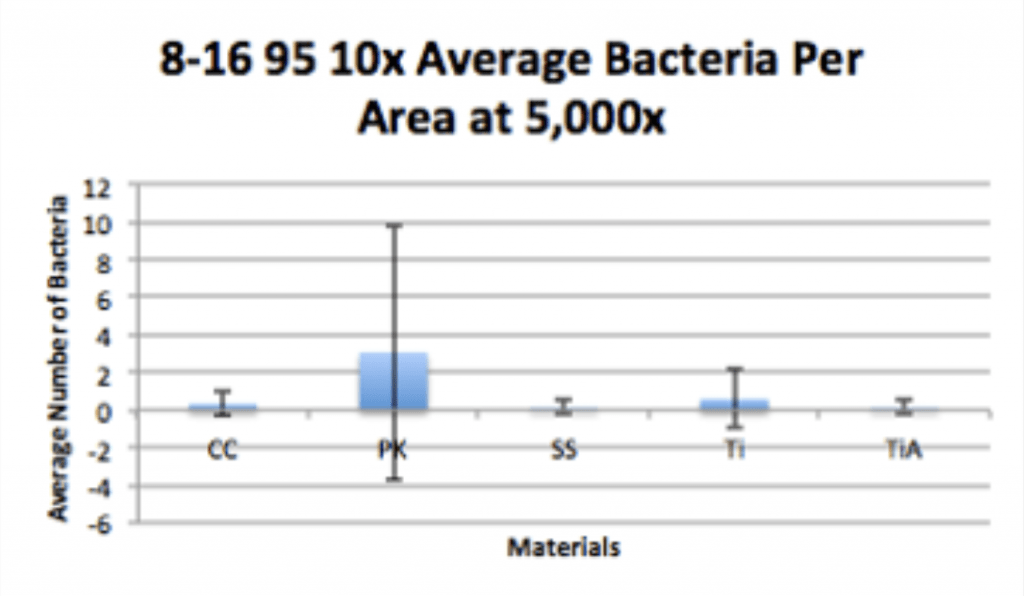
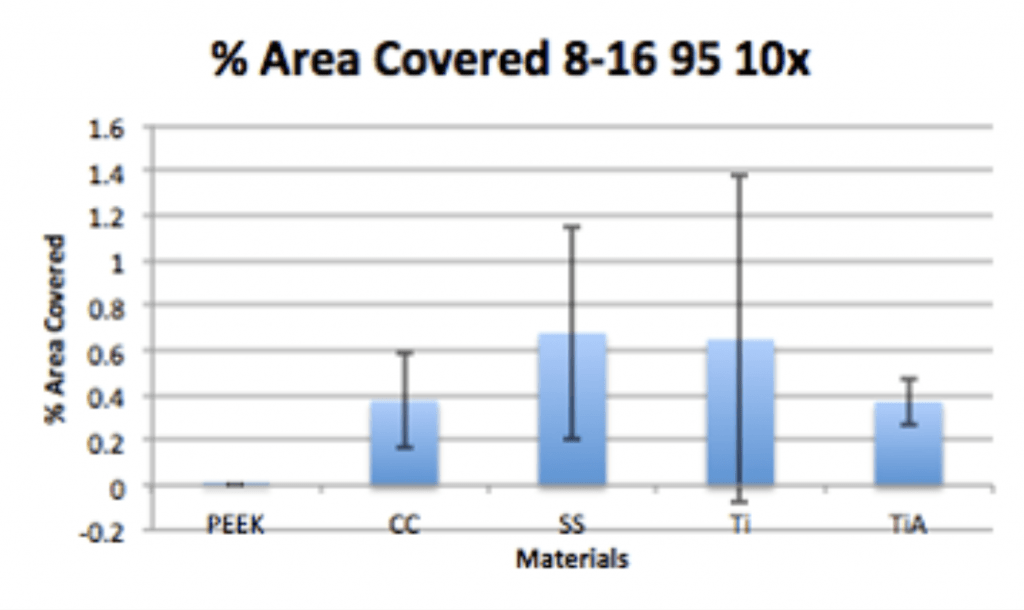

Conclusion
- P. acnes is able to form a biofilm within 4hrs of adherence and 20hrs of propagation on PEEK implants.
- Ag-Doped Coating is able to prevent biofilm formation and reduce overall bacterial adhesion.
- Ag-Doped Coating can penetrate deeper into pilosebaceous follicles than chlorheximide gluconate.