
By Mal Go
Illustration by Taimi Xu
Abstract
This narrative literature review explores the relationship between Gestational Diabetes Mellitus (GDM) and placental histopathology. The placenta, a crucial fetal organ, plays a vital role in maternal-fetal exchanges during pregnancy. GDM, a common complication affecting 2 to 10 percent of pregnancies in the US, is characterized by glucose intolerance. Placental hormones, particularly lactogen, influence maternal metabolic changes in GDM, leading to insulin resistance. However, the link between placental histopathology and GDM remains unclear. The review employed a narrative approach, searching databases for studies between 2010 and 2023. Sixteen quantitative studies focusing on the influence of GDM on placental histopathology were included. The studies varied in GDM definitions, diagnostic methods, and geographical locations. Placental histopathologic findings in GDM pregnancies included increased placental weight, chorangiosis, villous immaturity, and maternal vascular malperfusion. However, the understanding of these associations is limited, necessitating further research. Research gaps exist with only a few studies conducted in the USand limited exploration of maternal race as a potential risk factor. The need for tailored maternal recommendations and prevention strategies for adverse perinatal outcomes is emphasized. In conclusion, the review highlights the diverse placental histopathological characteristics associated with GDM. Future research should focus on larger prospective studies, exploring social determinants of health, non-clinical factors, and refining strategies for early detection and intervention to improve maternal and neonatal outcomes in GDM pregnancies.
Introduction
The human placenta, a transient fetal organ, serves as the interface between maternal and fetal circulatory systems. Formed from trophoblast cells, it consists of syncytiotrophoblast and cytotrophoblast layers, creating chorionic villi that connect to maternal blood.¹ As pregnancy progresses, cytotrophoblast decreases, facilitating maternal-fetal exchanges. The umbilical cord links to chorionic villi, the tiny, finger-like projections involved in uteroplacental circulation that promote the exchange of nutrients, oxygen, and waste between the mother and fetus.2 The placenta is vital for a healthy pregnancy, with functions such as implantation, blood circulation, hCG synthesis, hormone production, immune defense, and preventing immune rejection. Limited data on placental functions in fetal development exists, but abnormalities can lead to complications. Placental hormones mediate maternal adaptations, and issues may result in conditions like gestational diabetes and fetal abnormalities. Further research on placental pathology is crucial for a comprehensive understanding.
Gestational diabetes mellitus (GDM), one of the most common pregnancy complications, is defined as glucose intolerance that develops or is first recognized during pregnancy, and affects 2 to 10 percent of pregnancies in the US.3 The significant amount of the variability in GDM rates and prevalence between states can be attributed to racial/ethnic factors, maternal age, insurance at the individual level, hospital factors (type and bed count), and state-level factors (prevalence of obesity, income levels, poverty rates, etc).4 The hormone, lactogen, released by the placenta in pregnancy, is crucial for the promotion of maternal metabolic changes that occur during pregnancy to support the development and maintenance of the fetus.3 However, while lactogen is necessary for fetal nutrition, it is also an antagonist to insulin in pregnancy and promotes maternal insulin resistance. While the role of placental hormones and mechanisms in GDM is still unclear, less is known about the relationship between placental histopathology, specifically placental lesions, in pregnancies complicated with GDM, as well as the adverse pregnancy outcomes that may result from this histopathology.
This paper aims to synthesize current research regarding the placental histopathology of pregnancies complicated by GDM, identify limitations and strengths of current research, and recommend areas of improvement based on the literature. Throughout this paper, gendered language (“maternal”, “women”, etc) and non-gendered language (“people”, “persons”, “individuals”) will be used interchangeably with the intention to be inclusive of all identities while acknowledging that not all people who are pregnant or give birth identify themselves as women.
Search Methodology
Due to the broadness of placenta pathology and quantitative/qualitative gestational diabetes research literature, a narrative review approach was conducted. Systematized database searches were performed on November 30, 2023 for the time period of January 2010 to November 2023 and conducted using the databases Scopus, PubMed, Medline, Embase, and Cochrane for primary studies. Keywords searched included ‘placenta’, ‘placental histopathology’, ‘placenta pathology’, ‘gestational diabetes’, ‘abnormalities’, ‘adverse’, ‘neonatal’, ‘fetal’, and ‘vascular malperfusion’ (a complete list of search terms can be found in the appendix). Search terms were clustered according to the formatting requirements of each database to identify original, peer-reviewed research reports investigating the placental histopathology of gestational diabetes mellitus. Additional references were identified from a manual search of the cited references of research papers that were included from the initial search. The inclusion criteria for the full-text screening were English language or available translation, full-text availability, original research in peer-reviewed journals, published between 2010 and 2023, and described histopathological findings of pregnancies complicated by GDM. Gray literature and studies that did not clearly study the effect of GDM on placental histopathology were excluded. (See Table 1 for full search terms)
Table 1 Search Terms
| Cluster | Search Terms |
| A | GDM-related terms: Title or Abstract “gestational diabetes” OR GDM OR “perinatal diabetes” OR “maternal diabetes” OR “hyperglycemia in pregnancy” OR “diabetes in pregnancy” |
| B | placenta-related terms: Title or AbstractPlacental pathology OR placenta histopathology OR placenta abnormalities OR placental lesions OR placental dysfunction OR maternal vascular malperfusion |
| C | neonatal/fetal: Title or AbstractNeonatal complications OR neonatal adverse OR fetal adverse OR fetal complications |
| D | A, B, C |
Search Results
A total of 184 records were identified through database searching, and after removing duplicates 167 articles were screened against the eligibility criteria. Of these, 97 were excluded after screening the study title and abstract, leaving 70 full-text studies assessed for inclusion. A total of 16 quantitative studies that focused on the influence of GDM on placental histopathologic characteristics were eligible for inclusion and data extraction.
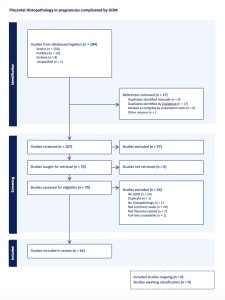
Figure 1 PRISMA: Search Flow-chart of identified papers published between 2010 and 2023.
Gestational Diabetes Mellitus diagnosis
All studies, except one that relied on medical records and patient charts for GDM diagnosis, used a variation of the oral glucose tolerance test (OGTT) for biochemically confirmed GDM diagnosis. Studies varied by the guidelines and criteria that the OGTT was scored against, including: International Association of the Diabetes and Pregnancy Study Groups (IADPSG), International Federation of Gynecology and Obstetrics (FIGO), American Diabetes Association (ADA), American College of Obstetricians and Gynecologists (ACOG), Carpenter-Coustan, and glucose challenge test (GCT, the UK version of the OGTT), departmental protocol, and Japanese criteria.
| Author, Year | Country | GDM definition | Type of Study | Retrospective/Prospective | GDM sample size | Blinding of Pathologist |
| Aldahmash, 2022 | Saudi Arabia | GDM diagnosed at 24–28 weeks of pregnancy according tothe criteria of the International Diabetes and PregnancyStudy Group (IADPSG) | Case-control | Retrospective | n=44 | Not mentioned |
| Al-Ofi, 2021 | Saudi Arabia | GDM diagnosed at 24–28 weeks of pregnancy according tothe criteria of the International Diabetes and PregnancyStudy Group (IADPSG) | Case-control | Retrospective | n=10 | Not mentioned |
| Berceanu, 2018 | Romania | Clinical diagnosis from medical records | Cohort | Prospective | n=16 | Not mentioned |
| Diceglie, 2021 | Italy | GDM diagnosed according to the International Federation of Gynecology and Obstetrics (FIGO) guidelines, by the Oral Glucose Tolerance Test (OGTT-75 g) performed at 24–28 weeks of gestation. | Case-control | Prospective | n=13 | Not mentioned |
| Ganer Herman, 2022 | Israel | GDM diagnosed at 24–28 weeks of pregnancy according to OGTT (GDMA1 was defined when sufficient glycemic control was achieved with no medical treatment;GDMA2 was defined as the need for medical treatment to achieve glycemic control during pregnancy) by abnormal value 1 hour after 50g OGTT or two pathological values in the 3 hour 100g OGTT | Cohort | Retrospective | n=668 | Yes |
| Goto, 2022 | Japan | GDM was diagnosed at 24–28 weeks of pregnancy according tothe criteria of the International Diabetes and PregnancyStudy Group (IADPSG) | Case-control | Retrospective | n=155 | Not mentioned |
| Huynh, 2015 | US | GDM was diagnosed by Carpenter-Coustan criteria by 100g OGTT | Case-control | Retrospective | n=126 | Not mentioned |
| Istrate-Ofiţeru, 2020 | Romania | World Health Organization (WHO) GDM definition by plasma glucose level | Case-control | Prospective | n=30 | Not mentioned |
| Kleiner, 2020 | Israel | Department Protocol by OGTT | Cohort | Retrospective | n=133 | Yes |
| Nataly, 2022 | Israel | OAV was diagnosed in one of the last three measurements on the 3 hour 100g OGTT, performed between 24 and 28 weeks of gestation. GDM diagnosed at 24–28 weeks of pregnancy according to OGTT (GDMA1 was defined when sufficient glycemic control was achieved with no medical treatment;GDMA2 was defined as the need for medical treatment to achieve glycemic control during pregnancy) | Case-control | Prospective | n=132 | Yes |
| Pooransari, 2020 | Iran | GDM was diagnosed either by 75g or by 100g OGTT | Case-control | Prospective | n=60 | Not mentioned |
| Rudge, 2011 | Brazil | GDM was diagnosed at 24–28 weeks of pregnancy according to the American Diabetes Association (ADA) criteria for the 100g OGTT | Cohort | Prospective | n=8 | Yes |
| Scifres, 2017 | US | GDM was diagnosed by Carpenter-Coustan criteria by 50g 1 hour OGTT, or two or more abnormal values by the 3 hour 100g OGTT | Cohort | Retrospective | n=1186 | No |
| Siassakos, 2022 | UK | All patients according to the local protocol had a random blood glucose (BG) in the first trimester, a glucose challenge test (GCT/OGTT) in the late second trimester and a third trimester 2 hour OGTT with 75g dependent on risk factors. | Case-control | Retrospective | n=80 | No |
| Weiner, 2018 | Israel | GDM was diagnosed at 24–28 weeks of pregnancy according to the American College of Obstetricians and Gynecologists guidelines for the 100g OGTT. GDM was defined as at least two pathological values on the OGTT. | Case-control | Retrospective | n=285 | Yes |
| Xu, 2021 | China | GDM was diagnosed at 24–28 weeks of pregnancy according to the American Diabetes Association (ADA) criteria for the 100g OGTT | Cohort | Retrospective | n=278 (with PEC) | Not mentioned |
Placental histopathologic findings
Prospective cohort studies
Berceanu et al. compared the morphology of placentas of Type 1 diabetes mellitus and GDM complicated pregnancies.5 Majority of the Type 1 diabetes mellitus and GDM cases presented with placentomegaly, an enlarged or oversized placenta, at the end of the third trimester with 40 percent of GDM cases presenting with immature appearance of the placenta. Villous immaturity, the underdevelopment of placental villi relative to gestational age, was found in 81.25 percent of GDM cases and chorangiosis in 37.5 percent.5
Rudge et al. compared the placental histopathology between placentas of pregnancies complicated by mild hyperglycemia (MGH), GDM, and pregestational diabetes.6 The placentas of GDM pregnancies had characteristics of delayed placental maturation (absence of syncytial nodes, high incidence of dysmaturity), yet 25 percent of neonates were classified as large for gestational age (LGA).6 Placental characteristics only observed in the GDM placentas included chorial and intimal edemas (lesions of circulatory pathology), Hofbauer cell hyperplasia (enlargement of fetal macrophages), and villitis (inflammatory lesion).¹
Retrospective cohort studies
Ganer Herman et al. investigated in vitro fertilization (IVF) pregnancies and unassisted complications by GDM and found that IVF neonates were more likely to suffer from one or more adverse outcomes such as respiratory distress syndrome.7 This was not explained by placental histology as there were no significant differences between IVF and unassisted pregnancies regarding maternal vascular malperfusion (MVM) lesions, abnormalities of the blood vessels supplying the placenta. Nor were there significant differences regarding fetal vascular malperfusion lesions (FVM), abnormalities of the blood vessels within the placenta that supply the fetus. Also, there were no significant differences between groups regarding the prevalence of histological chorioamnionitis, the inflammation of the membranes surrounding the fetus.
Kleiner et al. compared pregnancy outcomes and placental pathology among pregnancies complicated by macrosomia (neonatal birth weight of 4000g or greater), a condition associated with diabetic pregnancies, prolonged labor, shoulder dystocia, and neonatal hypoglycemia.8 Due to low sample size, GDM placentas and pre-pregnancy diabetes placentas were aggregated under the “diabetic macrosomia” group.8 The study concluded that the diabetic macrosomia group had mothers of more advanced age and higher BMI and did not differ significantly in terms of neonatal outcomes compared to the non-diabetic macrosomia group, though both groups had high rates of adverse neonatal outcomes (27.5 percent and 29 percent, respectively). Surprisingly, the placentas from the non-diabetic macrosomia group had greater rates of MVM and FVM and higher placental weights compared to the diabetic macrosomia group.
After adjusting for maternal age, race, educational attainment, and pre-pregnancy BMI across 1,186 placentas, Scifres et al. concluded that MVM lesions were the most common placental pathology among women with GDM, with respect to their retrospective cohort study.9 GDM is associated with excess gestational weight gain. This increases the risk for adverse pregnancy outcomes and may negatively impact various inflammatory processes that may occur in early gestation. While the mechanisms for the association between MVM and early gestational weight gain are unknown, Scifres et al. predicted that the “diminished early placentation seen with MVM” may impact the production of hormones that influence maternal metabolism.9 For example, the stronger early placentation of pregnancies not associated with any pathology, may lead to the increased production of hormones that are associated with morning sickness (nausea and vomiting) common in early gestation, mitigating early gestational weight gain.
Due to the association between GDM and PEC, Xu et al. explored the placental histopathological effects of preeclamptic pregnancies complicated by GDM (PE+GDM) compared to those without GDM (PE-GDM).10 PE+GDM placentas had significantly greater incidences of chorioamnionitis, MVM, and increased placenta weight. However, there were no significant differences regarding neonatal outcomes between the two groups.
Prospective case-control studies
Diceglie et al. reported that PHLPP1 (a phosphatase described to be involved in insulin resistance mechanisms) expression in placental tissues was increased in obese women with GDM compared to obese women without GDM.11 There is a lack of consensus on the role of PHLPP1 in GDM and its potential role as a regulator of an insulin feedback loop absent in diabetes.
Istrate-Ofiţeru et al. investigated the association between common pregnancy morbidities, GDM and gestational hypertension (GHTN) and placental morphopathological changes that influence fetal development.12 Both GDM and GHTN placentas had greater vascular density compared to placentas unassociated with any pathology, with the presence of chorangiosis slightly higher in GDM placentas than GHTN placentas. This study concludes that chorangiosis, a feature typical of GDM placental pathology, can also occur in pregnancies complicated with GHTN.
Nataly et al. compared pregnancy outcomes and placental pathologies of GDM pregnancies managed by diet-control (GDM1), GDM pregnancies managed by insulin, and cases that had one isolated abnormal value (OAV) regarding the OGTT.13 The fetal to placental birth weight ratio was lower among the GDMA2 group compared to the other study groups, but there were no significant differences between groups regarding MVM, acute inflammatory, or chronic villitis lesions. Similarly, Pooransari et al. found that well-controlled GDM (by either insulin or diet) had no significant differences in placental gross morphology and pregnancy outcome compared to pregnancies not associated with any pathology.14
Retrospective case-control studies
Aldahmash et al. found that the average placental weight of the GDM group was significantly higher than the control group.15 Within the GDM group, common vasculopathies on the maternal side of the placenta included villous agglutination and retroplacental hemorrhage. On the fetal side, the incidence of villous fibrinoid necrosis and chorangiosis was significantly higher within the GDM group compared to the control group. Chorangiosis is the excess of blood vessels in the placental villi and impacts fetoplacental circulation, compromising placenta function and glucose metabolism.
Al-Ofi et al. discovered that the serum levels of the angiogenic biomarkers VEGF, angiopoietin-2, endoglin, and endothelin-1 were significantly higher in GDM women compared to non-GDM women.16 The disruption of pro and anti-angiogenic biomarkers directly affect the development of feto-placental vessels and may be associated with GDM pathology. This study found that serum VEGF-A levels in GDM pregnant participants were significantly higher compared to non-GDM participants; these finds may explain the mechanisms underlying the impairments of the placental barrier in GDM that are associated with (abnormal placentation and the subsequent maternal and neonatal complications).16
On the other hand, Goto et al. found an increased incidence of fetal vascular malperfusion among GDM placentas than non-GDM placentas.17 However, this did not apply to maternal vascular malperfusion (MVM). While the exact mechanisms of fetal vascular malperfusion are unknown, the study’s findings suggest that maternal hyperglycemia may result in endothelial injury and dysfunction which results in the development of fetal vascular malperfusion.
Huynh et al. utilized multivariable logistic regression to compare placental pathologic characteristics between pregnancies complicated by T1DM, T2DM, and GDM.18 While there were no statistically significant differences in fetal thrombotic vasculopathy, GDM placentas had significantly more villous immaturity and T2DM placentas had a significantly higher rate of fetal acute chorioamnionitis. Maturational impairments like villous immaturity are associated with increased risk of perinatal mortality and chronic fetal hypoxia; inflammatory impairments like chorioamnionitis are associated with neonatal morbidities and mortalities and preterm delivery.19-21
Siassakos et al. aimed to study placental pathology of GDM as a risk factor of stillbirth by utilizing placental histopathology reports and clinical record analysis and focused on abnormal placental villous maturation, particularly distal villous immaturity (DVI).22 They discovered that, despite no formal diabetes diagnosis, significantly more women with disorders of villous maturation had at least one abnormal glucose test result compared to women without disorders of villous maturation. The prevalence of a formal GDM diagnosis did not differ significantly between various histopathologies.
Weiner et al. aimed to compare placental histopathological lesions and neonatal outcomes of pregnancies complicated by GDM between singleton and dichorionic diamniotic twin pregnancies.23 While MVM, villous immaturity, and LGA neonates were associated with singleton pregnancies, twin pregnancies resulted in significantly more NICU admissions.
Strengths and Limitations
The primary strengths of the studies included in this narrative literature review were the biochemical confirmation of GDM diagnoses by OGTT and the novelty of the groups involved such as IVF patients with GDM, PEC pregnancies complicated by GDM, macrosomic pregnancies, women with OAV, and singleton vs twin pregnancies. However, the majority of studies took place at a single center or university-affiliated hospital and acknowledged small sample size (overall and of the GDM subsample) as a limitation. Also, only 5 out of the 16 studies had blinded their pathologists to reduce potential biases, with 11 out of 16 studies either not mentioning the blinding of their pathologists or failing to include this in their methodology.
Gaps in the Research
Only 2 out of the 16 studies were conducted in the US (Scifres et al., 2017; Huynh et al., 2015) and only 2 studies looked at maternal race as a potential risk factor (Scifres et al., 2017; Huynh et al., 2015).9,18 However, due to sample size and available data, both studies aggregated maternal races into “White”, “Black”, and “Other”, or “White” and “non-White”, respectively. Scifres et al. highlighted the need to better understand placental function in order to tailor and individualize maternal recommendations and prevent adverse perinatal outcomes.9
Discussion and Implications for Future Placental Research
This narrative literature review on placental histopathology studies related to gestational diabetes mellitus (GDM) revealed various findings. “The placental histopathological characteristics that were significantly more frequent in pregnancies complicated by GDM included increased placental weight, incidence of chorangiosis, villous/placental immaturity, and MVM.”18 There was variation regarding the placental histopathological characteristics: villous agglutination, retroplacental hemorrhage, villous fibrinoid necrosis, increased angiogenic biomarkers, PHLPP1 expression, FVM, increased vascular density, and chorioamnionitis.
This review reveals a lack of clarity in understanding the link between placental histopathology and GDM, emphasizing the need for further research. The studies, conducted between 2010 and 2023, highlight variations in GDM definitions, diagnostic methods, and geographical locations. Notably, research gaps exist, with limited studies in the US and minimal exploration of maternal race as a potential risk factor. The call for tailored maternal recommendations and prevention strategies arises from the diverse placental histopathological characteristics associated with GDM. Future research should focus on larger prospective studies, considering social determinants of health, non-clinical factors, and refining strategies for early detection and intervention to improve maternal and neonatal outcomes in GDM pregnancies. Social determinants of health and non-clinical factors of interest include maternal ethnicity, culture, socioeconomic status, access to care, and lifestyle factors.
Pathology plays a critical role in public health as the study of disease at the diagnostic and etiologic level. While the studies reviewed were highly laboratory and clinical-based, they provide a critical component of public health by contributing to understanding the mechanisms behind disease, surveillance, and potential strategies for early detection.24-27 The reviewed studies emphasized the need for further research in the pathological field, particularly larger prospective studies, as well as the significance of prenatal care, perinatal glycemic control, the use of ultrasound to detect potential abnormalities, and lifestyle factors in improving maternal and neonatal outcomes in pregnancies complicated by GDM. The future of placental histopathology research offers an opportunity to investigate the social determinants of health or non-clinical factors that may underlie these histopathological characteristics.26-30
References
- Pregnancy – Uterus, Placenta, Development | Britannica. Accessed December 10, 2023. https://www.britannica.com/science/pregnancy/The-uterus-and-the-development-of-the-placenta
- Kojima J, Ono M, Kuji N, Nishi H. Human Chorionic Villous Differentiation and Placental Development. Int J Mol Sci. 2022;23(14):8003. doi:10.3390/ijms23148003
- Quintanilla Rodriguez BS, Mahdy H. Gestational Diabetes. In: StatPearls. StatPearls Publishing; 2023. Accessed December 10, 2023. http://www.ncbi.nlm.nih.gov/books/NBK545196/
- Matoba N, Mestan KK, Collins JW. Understanding Racial Disparities of Preterm Birth Through the Placenta. Clinical Therapeutics. 2021;43(2):287-296. doi:10.1016/j.clinthera.2020.12.013
- Berceanu C, Tetileanu AV, Ofi AM, et al. Morphological and ultrasound findings in the placenta of diabetic pregnancy.
- Rudge MV, Lima CP, Damasceno DC, et al. Histopathological placental lesions in mild gestational hyperglycemic and diabetic women. Diabetol Metab Syndr. 2011;3(1):19. doi:10.1186/1758-5996-3-19
- Ganer Herman H, Marom O, Koren L, et al. Gestational diabetes mellitus in in-vitro fertilization pregnancies – Clinical and placental histological characteristics. Placenta. 2022;117:156-160. doi:10.1016/j.placenta.2021.12.012
- Kleiner I, Ram S, Kovo M, et al. Pregnancy outcomes in association with placental histopathology in pregnancies complicated by macrosomia in diabetic vs. non-diabetic women. European Journal of Obstetrics & Gynecology and Reproductive Biology. 2020;248:24-29. doi:10.1016/j.ejogrb.2020.03.019
- Scifres CM, Parks WT, Feghali M, Caritis SN, Catov JM. Placental maternal vascular malperfusion and adverse pregnancy outcomes in gestational diabetes mellitus. Placenta. 2017;49:10-15. doi:10.1016/j.placenta.2016.11.004
- Xu F, Yang S, Liu Y, et al. Placental pathology and neonatal outcomes in pre-eclampsia with gestational diabetes mellitus. The Journal of Maternal-Fetal & Neonatal Medicine. 2021;34(7):1149-1154. doi:10.1080/14767058.2020.1786513
- Diceglie C, Anelli GM, Martelli C, et al. Placental Antioxidant Defenses and Autophagy-Related Genes in Maternal Obesity and Gestational Diabetes Mellitus. Nutrients. 2021;13(4):1303. doi:10.3390/nu13041303
- Istrate-Ofiţeru AM, Berceanu C, Berceanu S, et al. The influence of gestational diabetes mellitus (GDM) and gestational hypertension (GH) on placental morphological changes. Rom J Morphol Embryol. 2020;61(2):371-384. doi:10.47162/RJME.61.2.07
- Nataly F, Hadas GH, Ohad G, Letizia S, Michal K. Is there a difference in placental pathology in pregnancies complicated with gestational diabetes A2 versus gestational diabetes A1, versus one abnormal value, on 100 gr glucose tolerance test? Placenta. 2022;120:60-64. doi:10.1016/j.placenta.2022.02.009
- Pooransari P, Ebrahimi A, Nazemi N, Yaminifar F, Abediasl Z. Is gross morphology of placenta, umbilical cord, and neonatal outcome in well-controlled gestational diabetes mellitus pregnancy different? A case-control study. IJRM. Published online July 2, 2020. doi:10.18502/ijrm.v13i6.7282
- Aldahmash WM, Alwasel SH, Aljerian K. Gestational diabetes mellitus induces placental vasculopathies. Environ Sci Pollut Res. 2022;29(13):19860-19868. doi:10.1007/s11356-021-17267-y
- Al-Ofi E, Alrafiah A, Maidi S, Almaghrabi S, Hakami N. Altered Expression of Angiogenic Biomarkers in Pregnancy Associated with Gestational Diabetes. IJGM. 2021;Volume 14:3367-3375. doi:10.2147/IJGM.S316670
- Goto T, Sato Y, Kodama Y, et al. Association between fetal vascular malperfusion and gestational diabetes. J of Obstet and Gynaecol. 2022;48(1):80-86. doi:10.1111/jog.15046
- Huynh J, Yamada J, Beauharnais C, et al. Type 1, type 2 and gestational diabetes mellitus differentially impact placental pathologic characteristics of uteroplacental malperfusion. Placenta. 2015;36(10):1161-1166. doi:10.1016/j.placenta.2015.08.004
- Burton GJ, Fowden AL. The placenta: a multifaceted, transient organ. Philos Trans R Soc Lond B Biol Sci. 2015;370(1663):20140066. doi:10.1098/rstb.2014.0066
- Heazell A. The placenta and adverse pregnancy outcomes – opening the black box? BMC Pregnancy and Childbirth. 2015;15(1):A5. doi:10.1186/1471-2393-15-S1-A5
- Jauniaux E. Early Placentation Disorders. EGA Institute for Women’s Health. Published October 28, 2019. Accessed December 10, 2023. https://www.ucl.ac.uk/womens-health/research/reproductive-health/early-placentation-disorders
- Siassakos D, Bourne I, Sebire N, Kindinger L, Whitten SM, Battaglino C. Abnormal placental villous maturity and dysregulated glucose metabolism: implications for stillbirth prevention. Journal of Perinatal Medicine. 2022;50(6):763-768. doi:10.1515/jpm-2021-0579
- Weiner E, Barber E, Feldstein O, et al. The placental component and neonatal outcome in singleton vs. twin pregnancies complicated by gestational diabetes mellitus. Placenta. 2018;63:39-44. doi:10.1016/j.placenta.2018.01.010
- Kingdom J, Hutcheon JA, Gordijn SJ, El-Demellawy D, Grynspan D. Placental Pathology and Pregnancy Complications. Journal of Clinical Medicine. 2023;12(15). doi:10.3390/jcm12155053
- Loverro MT, Di Naro E, Nicolardi V, et al. Pregnancy Complications, Correlation With Placental Pathology and Neonatal Outcomes. Frontiers in Clinical Diabetes and Healthcare. 2022;2. Accessed December 10, 2023. https://www.frontiersin.org/articles/10.3389/fcdhc.2021.807192
- Matoba N, Mestan KK, Collins JW. Understanding Racial Disparities of Preterm Birth Through the Placenta. Clinical Therapeutics. 2021;43(2):287-296. doi:10.1016/j.clinthera.2020.12.013
- Matoba N, Yallapragada S, Davis MM, Ernst LM, Collins JW, Mestan KK. Racial differences in placental pathology among very preterm births. Placenta. 2019;83:37-42. doi:10.1016/j.placenta.2019.06.385
- Min AM, Saito M, Simpson JA, Kennedy SH, Nosten FH, McGready R. Placental histopathology in preterm birth with confirmed maternal infection: A systematic literature review. PLoS ONE. 2021;16(8). doi:10.1371/journal.pone.0255902
- Thornburg KL, Boone-Heinonen J, Valent AM. Social Determinants of Placental Health and Future Disease Risks for Baby. Obstetrics and gynecology clinics of North America. 2020;47(1):1. doi:10.1016/j.ogc.2019.11.002
- Zhang P, Dygulski S, Al-Sayyed F, Dygulska B, Lederman S. Differences in Prevalence of Pregnancy Complications and Placental Pathology by Race and Ethnicity in a New York Community Hospital. JAMA Netw Open. 2022;5(5):e2210719. doi:10.1001/jamanetworkopen.2022.10719
- Alugupalli K. Role of Pathology in Public Health. J Pathol Dis Biol. 2023;7(4):156
- Audette MC, Levytska K, Lye SJ, Melamed N, Kingdom JC. Parental ethnicity and placental maternal vascular malperfusion pathology in healthy nulliparous women. Placenta. 2018;66:40-46. doi:10.1016/j.placenta.2018.04.014
- Bentley-Lewis R, Dawson DL, Wenger JB, Thadhani RI, Roberts DJ. Placental Histomorphometry in Gestational Diabetes Mellitus: The Relationship Between Subsequent Type 2 Diabetes Mellitus and Race/Ethnicity. American Journal of Clinical Pathology. 2014;141(4):587-592. doi:10.1309/AJCPX81AUNFPOTLL
- Chen Y, Huang L, Zhang H, Klebanoff M, Yang Z, Zhang J. Racial disparity in placental pathology in the collaborative perinatal project. Int J Clin Exp Pathol. 2015;8(11):15042-15054.
- Dube YP, Nyapwere N, Magee LA, et al. Interactions between the Physical and Social Environments with Adverse Pregnancy Events Related to Placental Disorders—A Scoping Review. International Journal of Environmental Research and Public Health. 2020;17(15). doi:10.3390/ijerph17155421
- Ducatman BS, Ducatman AM, Crawford JM, Laposata M, Sanfilippo F. The Value Proposition for Pathologists: A Population Health Approach. Academic Pathology. 2020;7. doi:10.1177/2374289519898857
- Kawamura MY, Mau MK, Soon R, Yamasato K. A Scoping Review on Gestational Diabetes in Hawai‘i: A “Window of Opportunity” to Address Intergenerational Risk for Type 2 Diabetes Mellitus. Hawaii J Health Soc Welf. 2022;81(3):58-70.
- Lett E, Asabor E, Beltrán S, Cannon AM, Arah OA. Conceptualizing, Contextualizing, and Operationalizing Race in Quantitative Health Sciences Research. Annals of Family Medicine. 2022;20(2):157. doi:10.1370/afm.2792