Class: CHEM 0500 Inorganic Chemistry
Instructor(s): Dr. Eric Victor
Student(s): Sharon Lee (Chemistry ‘20), Stephanie Adaniya, Zachary Burger, Arvind Veluvali
Description:
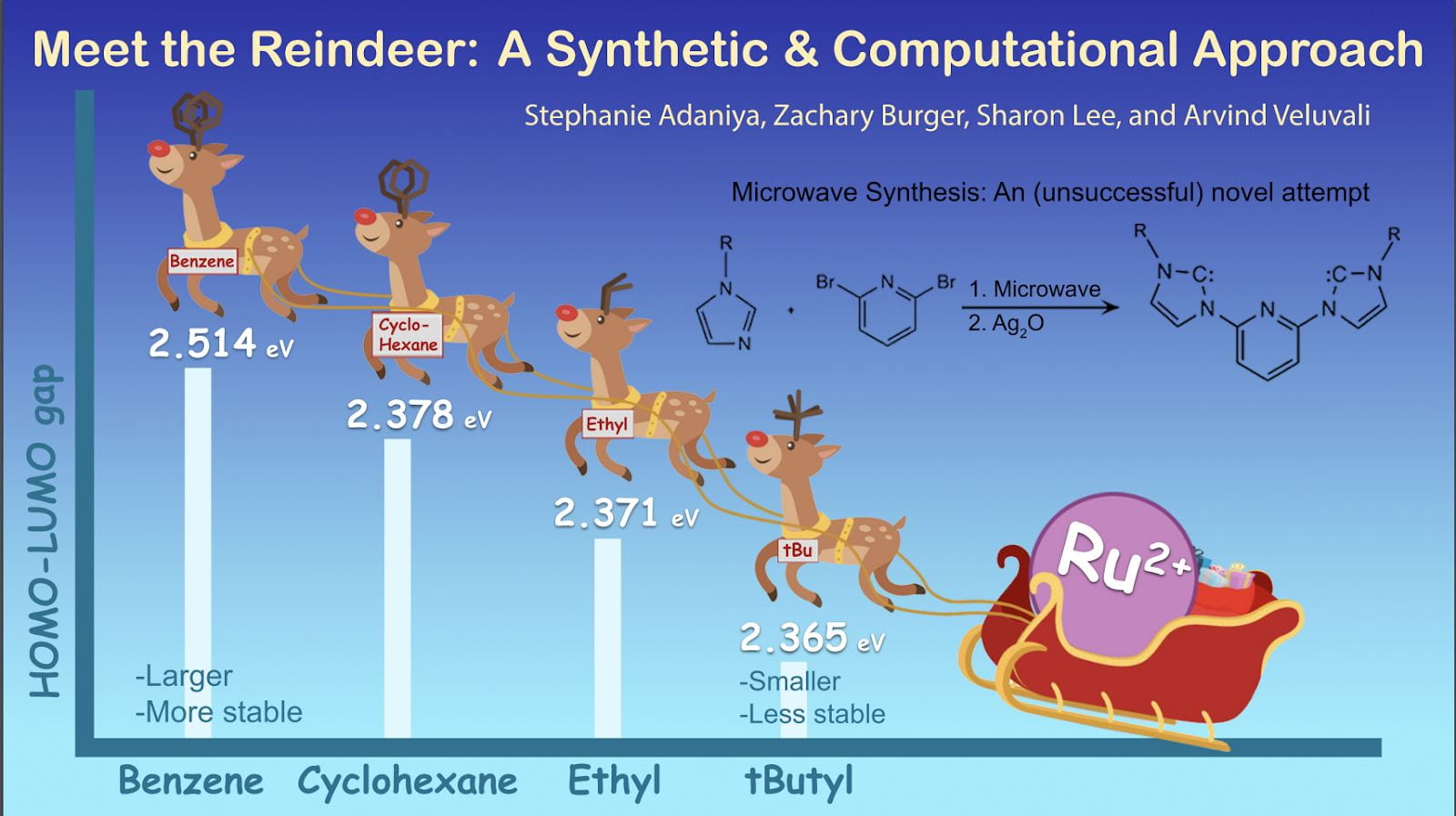
A comprehensive two-part analysis of a ligand (“reindeer”) binding to an octahedral ruthenium (II) complex (RuIIL2) revealed how properties such as bond length and angle, IR spectra, 1H NMR, UV-Vis spectra, and molecular orbitals change as the R-group of the reindeer is substituted with ethyl, t-butyl, cyclohexane, and benzene functional groups. Notable findings include the following: Ru complexes with larger ligand molecular weight and size had the highest HOMO-LUMO gap and were therefore the most stable; bond length, angle, and overall geometry were largely determined by R-group steric hindrance; and there was a strong correlation between the physical properties of free ligand and bound ligand. The incorporation of CURE into the class curriculum has made research more accessible and less intimidating.